Everything You Need To Know About Gut Healing Diets
How To Heal Leaky Gut, Gut Symptoms, and Calm Inflammation With the Right Diet
- What Is a Gut-Healing Diet?|
- Why Use One?|
- Best Gut Diet|
- FODMAP|
- Paleo|
- Other Diets|
- How to Eat A Gut Healing Diet|
- What to Include|
- Where To Go From Here|
Download this Episode (right click link and ‘Save As’)
Your gut isn’t just for digesting. Making sure your gut is in good health can not only improve your digestive symptoms, but your mood, sleep, and skin as well. Making the right food choices for your body can allow your gut to heal. Though there are many possible gut healing diets, a few particular diets are known for their positive impact on digestive symptoms.
In this article, we’ll discuss what makes a gut healing diet, its benefits, the best gut healing diet options, and how to follow your gut healing diet.

What Is a Gut Healing Diet?
When it comes to the digestive tract, inflammation equals symptoms. Gut inflammation and intestinal permeability are known to trigger or contribute to many health challenges [1, 2, 3, 4, 5].
A gut healing diet is any diet you adopt to reduce digestive symptoms, inflammation, or other related symptoms, such as joint pain, skin symptoms, or insomnia, for example.
Because many common foods can inflame the gut, gut healing diets are usually elimination diets. An elimination diet removes likely trigger foods so you can evaluate what exacerbates your symptoms. Removing the problem foods also reduces inflammation, which gives the gut a chance to heal and repair.
A gut healing elimination diet is designed to:
- Reduce or remove foods that may irritate your gut lining or feed imbalanced gut bacteria.
- Heal and seal intestinal permeability (leaky gut)
- Feed your good gut bacteria
- Reduce inflammation
- Help you understand which foods are triggering your symptoms
Why Would You Use A Gut Healing Diet?
A gut healing diet is one of the fastest ways to relieve the worst immediate symptoms of your health challenges. Here is a list of symptoms or conditions that may benefit from a gut healing diet.
Digestive symptoms and conditions:
- Chronic constipation or diarrhea
- Bloating, abdominal pain, or gas
- Food allergies or food sensitivities
- Heartburn or reflux
- Irritable Bowel Syndrome (IBS)
- Inflammatory Bowel Disease (IBD), such as Crohn’s disease or ulcerative colitis
- Small Intestinal Bacterial Overgrowth (SIBO)
- Celiac disease
Non-digestive issues:
- Autoimmune Diseases, such as Hashimoto’s Thyroiditis or Type 1 Diabetes
- Skin symptoms such as acne, rosacea, eczema, or psoriasis
- Mood symptoms, such as anxiety or depression
- Hypothyroidism Thyroid
- Medication malabsorption
- Fatigue
- Headaches and migraines
- Joint and muscle pain
Leaky Gut and Gut Health
Many health conditions relate to the gut through intestinal permeability. Intestinal permeability — otherwise known as leaky gut — has been documented in a wide range of health issues, such as digestive conditions [6, 7, 8, 9], mental health conditions [10, 11, 12, 13, 14, 15, 16, 17, 18, 19], fatigue [20, 21, 22] and autoimmune diseases [23, 24, 25, 26, 27, 28, 29].

Intestinal permeability is when small gaps open up the tight junctions between the cells of the gut lining in the small intestine. This allows partially digested food particles to enter the bloodstream, which can trigger an immune system response. Normally, the gut barrier prevents these food particles from entering the body until they are broken down further.
Gut healing diets, such as the low FODMAP diet [30, 31] have been documented to improve inflammation and gastrointestinal symptoms, which likely means they improve leaky gut as well.
What’s the Best Gut Diet?
The best gut healing diet for you will be highly individualized. That said, there are some basic principles of a healthy diet that apply to everyone.

This means that to maintain a healthy gut, you should generally aim to eat an anti-inflammatory diet that:
- Is made primarily of real, whole foods
- Is free of sweeteners and processed foods
- Contains the right ratio of carbs, healthy fats, quality proteins, and fiber for you
- Avoids your unique food sensitivities and food allergies
In addition, your best gut healing diet also needs to have a positive effect on your gut microbiome.
A few particular elimination diets meet these criteria. Let’s review which specific diets have been shown to improve digestive health and reduce inflammation and symptoms in research.
Low FODMAP Diet
FODMAPs are fermentable carbohydrates that naturally occur in some veggies, fruits, nuts, and seeds. Many people with digestive symptoms and disorders are sensitive to FODMAPs, so a low FODMAP diet can help reduce symptoms. A low FODMAP diet has been shown to:
- Improve diarrhea in IBS-D patients [32]
- Improve IBS symptoms in patients with inflammatory bowel syndrome (IBS) [33, 34, 35, 36]
- Improve gut endocrine cells, which can normalize bowel function [37, 38, 39]
- Improve histamine levels [40] (though this study was recently challenged)
- Reduce digestive symptoms, such as bloating, abdominal pain, and gas [41, 42]
- Improve intestinal permeability (leaky gut) [43, 44]
For these reasons, the low FODMAP diet is one of the diets we recommend most frequently in our clinic.
Paleo Diet
The paleo diet is a basic, low carb elimination diet that improves inflammation by minimizing your exposure to foods that may provoke an immune response [45, 46]. These include sugars, unhealthy fats, chemical additives, and common problem foods like dairy, gluten, and soy.
The principles of avoiding processed foods is one of the foundations of conventional IBS diet advice. Preliminary research suggests that the paleo diet reduces digestive symptoms [47]. It’s also been documented to reduce inflammation and inflammatory conditions, including diabetes and heart disease [48, 49, 50]. Reducing inflammation creates a better environment for gut bacteria and can improve your gut microbiome [51, 52]. This can also reduce digestive symptoms, which are often linked to an overgrowth of the wrong kind of gut bacteria [53, 54, 55, 56].
Other Specialty Gut Healing Diets
There are many other special dietary plans that can help address digestive symptoms. But these plans should only be used if more basic plans haven’t provided the results you’re looking for.
An elemental diet is a meal-replacement shake that contains pre-digested “elemental” nutrition, which means there is no fiber to irritate the digestive system. Elemental diets have been shown to improve IBD symptoms [57, 58, 59, 60, 61, 62, 63], SIBO [64], and celiac disease [65] in clinical trials. An elemental diet is useful as a short-term gut reset, during a symptom flare up, or as a regular meal replacement to rest the digestion on a day-to-day basis.
The low-histamine diet decreases or removes foods high in histamine, or foods that increase the release of histamine. Histamine intolerance is one possible reason for digestive symptoms such as diarrhea, nausea and vomiting, bloating, and gas. Decreasing dietary histamine has been shown to reduce histamine intolerance symptoms [66]. FODMAPs may feed the bacteria that often trigger histamine intolerance, so be sure to diligently use the low FODMAP diet before testing a low histamine diet [67, 68, 69, 70].
The Autoimmune Paleo Diet (AIP) removes additional possible immune triggers beyond the basic paleo diet (including eggs, nuts, seeds, and nightshade vegetables such as tomatoes, eggplant, potatoes, and peppers). AIP has been shown in a few early studies to improve IBD symptoms [71, 72, 73] so may have some value as a gut healing diet if you haven’t seen improvement with these other diets.
How To Eat a Gut Healing Diet

Making any kind of dietary change can be a challenge, so make it easy on yourself by following these guidelines while you make the transition:
- Keep it simple. Choose a few basic recipes and use them to develop a simple meal plan. Expand your repertoire once you’re comfortable with your new diet.
- Be prepared. Shop for ingredients you need for your basic meal plan, and remove the foods you’ll be avoiding from your pantry. Batch cook your simple recipes, and load up your freezer.
- Be as strict as possible for 2-4 weeks. If you don’t notice any positive changes in that time, try something different.
- Use what you learn to create a sustainable, healthy diet going forward.
What To Include in a Gut Healing Diet
Now that you’ve removed inflammatory foods, it’s time to talk about what to add. No matter which gut healing diet template you choose to work with, including particular gut healing foods and supplements can increase your chances for success. Let’s review some of the options.
Probiotics and Prebiotics
Probiotic supplements have been shown in numerous studies to:
- Improve intestinal permeability [74, 75, 76, 77, 78]
- Decrease gut inflammation [79]
- Improve digestive symptoms, such as gas, bloating, abdominal pain, diarrhea, and constipation [80, 81, 82, 83, 84, 85, 86, 87, 88].
- Balance bacterial overgrowth and the overall gut flora [89, 90]
- Crowd out bad bacteria [91, 92, 93]
This makes them superstars of a gut healing diet.
A diversity of probiotic supplements seem to work better than single strains [94, 95]. For best results, choose one high quality product from each of the three categories — a Lactobacillus and Bifidobacteria blend, a Saccharomyces boulardii, and a soil-based probiotic — and use them together.
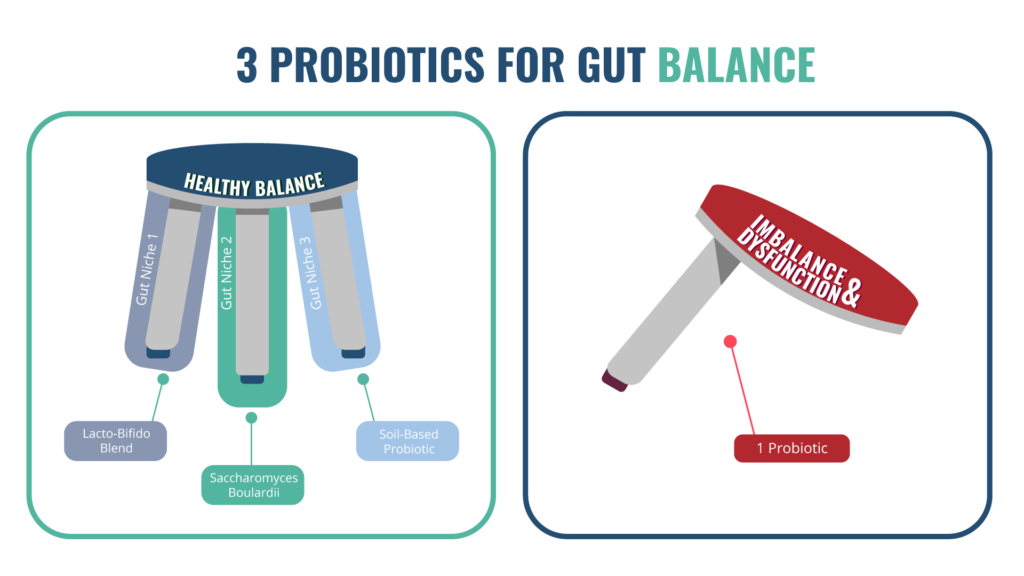
Prebiotics are simply natural fibers that feed your good gut bacteria. Most people get enough prebiotics from their diet, as long as it contains sufficient fruits, veggies, nuts, and seeds. However, some people need supplemental prebiotics to make their gut microbes happy. In spite of this, prebiotics may increase digestive symptoms for people, so they should be tried with caution [96].
Bone Broth and Collagen
Bone broth is naturally high in gelatin and collagen, which is made primarily of the amino acids glutamic acid, proline, and glycine [97]. Collagen has been shown to improve skin elasticity and hydration [98, 99, 100]. And though the data are early, some studies have suggested that collagen directly improves intestinal permeability [101]. And though these are lower quality data, other studies showed glutamic acid improved intestinal permeability in pigs [102] and L-glycine given to rats prevented ulcers [103].
Fortunately, you don’t need to try to use these amino acids separately. Homemade or commercially prepared bone broth can provide healthy doses of these gut healing nutrients, especially if consumed regularly.
Fermented Foods
Fermented foods are valuable for gut health, as they are a natural source of good bacteria. Though they don’t typically have therapeutic doses of probiotics [104, 105, 106, 107], they may continue to seed your digestive system with diverse probiotics from natural fermentation when used as a small, daily supplement. Examples include sauerkraut, kefir, kimchi, and kombucha.
Those with histamine intolerance often do not tolerate aged or fermented foods, so avoid if intolerant.
Gut Healing Supplements

Diet and probiotics should always be your first, go-to step to work on gut healing, but a number of gut healing supplements can enhance your progress once that piece is in place.
In general, give supplements a short-term trial to see if they improve your symptoms. If so, then continue using them. If not, you can discontinue them. Here are a few that are most likely to improve your gut healing diet success.
Digestive Enzymes
Digestive enzymes are chemicals your body naturally produces to break down carbohydrates, fats, and proteins. A deficiency of particular enzymes can lead to digestive symptoms.
For example, if you don’t make lactase, the enzyme responsible for breaking down lactose in dairy products, you’ll experience lactose intolerance symptoms like gas, bloating, and diarrhea.
There is some early evidence indicating that taking digestive enzymes can help improve digestive symptoms, such as bloating and abdominal pain [108, 109, 110, 111].
Stomach Acid Supplementation (Betaine HCl)
Low stomach acid is associated with a wide variety of health problems, including SIBO [112], H. pylori infection [113], autoimmune conditions [114, 115, 116, 117, 118, 119, 120, 121, 122], and anemia [123].
There isn’t yet much research into the use of betaine HCl, but two studies show it’s effective for increasing stomach acid, acts quickly, and has a short-term effect of just over an hour [124, 125]. Including supplemental stomach acid may improve your digestion. Betaine HCl should be avoided if you have peptic ulcers.
L-Glutamine
L-glutamine is an amino acid your body naturally produces to repair your gut lining. L-glutamine has been shown in multiple studies, including meta-analyses, to improve intestinal permeability and inflammation, and reduce digestive symptoms [126, 127, 128, 129].
Where To Go From Here
Eating a gut healing diet is mostly about common sense. Eat a healthy, anti-inflammatory diet full of nutrition, and watch how quickly your gut and other health challenges respond. Include supportive supplements if needed.
For more support starting your gut healing diet, consider reading my book, Healthy Gut, Healthy You, or meeting with a health coach or nutritionist at our newly opened Ruscio Institute for Functional Medicine.
Dr. Michael Ruscio is a DC, natural health provider, researcher, and clinician. He serves as an Adjunct Professor at the University of Bridgeport and has published numerous papers in scientific journals as well as the book Healthy Gut, Healthy You. He also founded the Ruscio Institute of Functional Health, where he helps patients with a wide range of GI conditions and serves as the Head of Research.➕ References
- Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014 Jan 7;20(1):91-9. doi: 10.3748/wjg.v20.i1.91. PMID: 24415861; PMCID: PMC3886036.
- Coates LC, FitzGerald O, Helliwell PS, Paul C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same? Semin Arthritis Rheum. 2016 Dec;46(3):291-304. doi: 10.1016/j.semarthrit.2016.05.012. Epub 2016 Jun 2. PMID: 27388027.
- Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, Flores-Ramírez R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018 Nov;27(11):1327-1334. doi: 10.1016/j.hlc.2018.05.102. Epub 2018 Jun 2. PMID: 29903685.
- Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018 Jan 8;360:j5145. doi: 10.1136/bmj.j5145. PMID: 29311119; PMCID: PMC6889978.
- Shah PK, Lecis D. Inflammation in atherosclerotic cardiovascular disease. F1000Res. 2019 Aug 9;8:F1000 Faculty Rev-1402. doi: 10.12688/f1000research.18901.1. PMID: 31448091; PMCID: PMC6694447.
- Mujagic Z, Ludidi S, Keszthelyi D, Hesselink MA, Kruimel JW, Lenaerts K, Hanssen NM, Conchillo JM, Jonkers DM, Masclee AA. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther. 2014 Aug;40(3):288-97. doi: 10.1111/apt.12829. Epub 2014 Jun 18. PMID: 24943095.
- Michielan A, D’Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015:628157. doi: 10.1155/2015/628157. Epub 2015 Oct 25. PMID: 26582965; PMCID: PMC4637104.
- Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015 Jan;13(1):11-8. doi: 10.5217/ir.2015.13.1.11. Epub 2015 Jan 29. PMID: 25691839; PMCID: PMC4316216.
- Chang J, Leong RW, Wasinger VC, Ip M, Yang M, Phan TG. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology. 2017 Sep;153(3):723-731.e1. doi: 10.1053/j.gastro.2017.05.056. Epub 2017 Jun 8. PMID: 28601482.
- Karakula-Juchnowicz H, Rog J, Juchnowicz D, Łoniewski I, Skonieczna-Żydecka K, Krukow P, Futyma-Jedrzejewska M, Kaczmarczyk M. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): a 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr J. 2019 Aug 31;18(1):50. doi: 10.1186/s12937-019-0475-x. PMID: 31472678; PMCID: PMC6717641.
- Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012 Dec 1;141(1):55-62. doi: 10.1016/j.jad.2012.02.023. Epub 2012 Mar 11. PMID: 22410503.
- Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF. In Schizophrenia, Increased Plasma IgM/IgA Responses to Gut Commensal Bacteria Are Associated with Negative Symptoms, Neurocognitive Impairments, and the Deficit Phenotype. Neurotox Res. 2019 Apr;35(3):684-698. doi: 10.1007/s12640-018-9987-y. Epub 2018 Dec 15. PMID: 30552634.
- 1. Wright ML, Fournier C, Houser MC, Tansey M, Glass J, Hertzberg VS. Potential Role of the Gut Microbiome in ALS: A Systematic Review. Biological Research For Nursing. 2018;20(5):513-521. doi:10.1177/1099800418784202
- Nguyen TT, Kosciolek T, Eyler LT, Knight R, Jeste DV. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J Psychiatr Res. 2018 Apr;99:50-61. doi: 10.1016/j.jpsychires.2018.01.013. Epub 2018 Jan 31. PMID: 29407287; PMCID: PMC5849533.
- Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008 Feb;29(1):117-24. PMID: 18283240.
- Dutta SK, Verma S, Jain V, Surapaneni BK, Vinayek R, Phillips L, Nair PP. Parkinson’s Disease: The Emerging Role of Gut Dysbiosis, Antibiotics, Probiotics, and Fecal Microbiota Transplantation. J Neurogastroenterol Motil. 2019 Jul 1;25(3):363-376. doi: 10.5056/jnm19044. PMID: 31327219; PMCID: PMC6657920.
- Maguire M, Maguire G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev Neurosci. 2019 Jan 28;30(2):179-201. doi: 10.1515/revneuro-2018-0024. PMID: 30173208.
- Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 2011;6(12):e28330. doi: 10.1371/journal.pone.0028330. Epub 2011 Dec 2. PMID: 22164271; PMCID: PMC3229570.
- Buscarinu MC, Romano S, Mechelli R, Pizzolato Umeton R, Ferraldeschi M, Fornasiero A, Reniè R, Cerasoli B, Morena E, Romano C, Loizzo ND, Umeton R, Salvetti M, Ristori G. Intestinal Permeability in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics. 2018 Jan;15(1):68-74. doi: 10.1007/s13311-017-0582-3. PMID: 29119385; PMCID: PMC5794695.
- Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. 2008 Dec;29(6):902-10. PMID: 19112401.
- Maes M, Coucke F, Leunis JC. Normalization of the increased translocation of endotoxin from gram negative enterobacteria (leaky gut) is accompanied by a remission of chronic fatigue syndrome. Neuro Endocrinol Lett. 2007 Dec;28(6):739-44. PMID: 18063928.
- Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008 Feb;29(1):117-24. PMID: 18283240.
- Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106:16799–804.
- Morris G, Berk M, Carvalho AF, Caso JR, Sanz Y, Maes M. The Role of Microbiota and Intestinal Permeability in the Pathophysiology of Autoimmune and Neuroimmune Processes with an Emphasis on Inflammatory Bowel Disease Type 1 Diabetes and Chronic Fatigue Syndrome. Curr Pharm Des. 2016;22(40):6058-6075. doi: 10.2174/1381612822666160914182822. PMID: 27634186.
- Bjarnason I, Williams P, So A, Zanelli GD, Levi AJ, Gumpel JM, Peters TJ, Ansell B. Intestinal permeability and inflammation in rheumatoid arthritis: effects of non-steroidal anti-inflammatory drugs. Lancet. 1984 Nov 24;2(8413):1171-4. doi: 10.1016/s0140-6736(84)92739-9. PMID: 6150232.
- Goebel A, Buhner S, Schedel R, Lochs H, Sprotte G. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology (Oxford). 2008 Aug;47(8):1223-7. doi: 10.1093/rheumatology/ken140. Epub 2008 Jun 7. PMID: 18540025.
- Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016 Oct 21;4(4):e1251384. doi: 10.1080/21688370.2016.1251384. PMID: 28123927; PMCID: PMC5214347.
- Küçükemre Aydın B, Yıldız M, Akgün A, Topal N, Adal E, Önal H. Children with Hashimoto’s Thyroiditis Have Increased Intestinal Permeability: Results of a Pilot Study. J Clin Res Pediatr Endocrinol. 2020 Sep 2;12(3):303-307. doi: 10.4274/jcrpe.galenos.2020.2019.0186. Epub 2020 Jan 28. PMID: 31990165; PMCID: PMC7499128.
- Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D’Agate C, Not T, Zampini L, Catassi C, Fasano A. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006 Apr;41(4):408-19. doi: 10.1080/00365520500235334. PMID: 16635908.
- Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010 Feb;25(2):252-8. doi: 10.1111/j.1440-1746.2009.06149.x. PMID: 20136989.
- Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012 May;107(5):657-66; quiz 667. doi: 10.1038/ajg.2012.49. Epub 2012 Apr 10. PMID: 22488077.
- Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled trial. J Gastroenterol Hepatol. 2018 Jun;33(6):1192-1199. doi: 10.1111/jgh.14051. Epub 2018 Feb 21. PMID: 29159993.
- Gibson PR. Use of the low-FODMAP diet in inflammatory bowel disease. J Gastroenterol Hepatol. 2017 Mar;32 Suppl 1:40-42. doi: 10.1111/jgh.13695. PMID: 28244679.
- Pedersen N, Ankersen DV, Felding M, Wachmann H, Végh Z, Molzen L, Burisch J, Andersen JR, Munkholm P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J Gastroenterol. 2017 May 14;23(18):3356-3366. doi: 10.3748/wjg.v23.i18.3356. PMID: 28566897; PMCID: PMC5434443.
- Zhan YL, Zhan YA, Dai SX. Is a low FODMAP diet beneficial for patients with inflammatory bowel disease? A meta-analysis and systematic review. Clin Nutr. 2018 Feb;37(1):123-129. doi: 10.1016/j.clnu.2017.05.019. Epub 2017 May 24. PMID: 28587774.
- Cox SR, Lindsay JO, Fromentin S, Stagg AJ, McCarthy NE, Galleron N, Ibraim SB, Roume H, Levenez F, Pons N, Maziers N, Lomer MC, Ehrlich SD, Irving PM, Whelan K. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology. 2020 Jan;158(1):176-188.e7. doi: 10.1053/j.gastro.2019.09.024. Epub 2019 Oct 2. PMID: 31586453.
- Mazzawi T, El-Salhy M. Changes in duodenal enteroendocrine cells in patients with irritable bowel syndrome following dietary guidance. Exp Biol Med (Maywood). 2017 Jul;242(13):1355-1362. doi: 10.1177/1535370217699537. Epub 2017 Mar 17. PMID: 28737477; PMCID: PMC5528200.
- Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur J Clin Nutr. 2016 Feb;70(2):175-81. doi: 10.1038/ejcn.2015.191. Epub 2015 Nov 25. PMID: 26603880; PMCID: PMC4744244.
- Mazzawi T, El-Salhy M. Effect of diet and individual dietary guidance on gastrointestinal endocrine cells in patients with irritable bowel syndrome (Review). Int J Mol Med. 2017 Oct;40(4):943-952. doi: 10.3892/ijmm.2017.3096. Epub 2017 Aug 11. PMID: 28849091; PMCID: PMC5593462.
- McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017 Jul;66(7):1241-1251. doi: 10.1136/gutjnl-2015-311339. Epub 2016 Mar 14. Erratum in: Gut. 2019 Jul;68(7):1342. PMID: 26976734.
- Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010 Feb;25(2):252-8. doi: 10.1111/j.1440-1746.2009.06149.x. PMID: 20136989.
- Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012 May;107(5):657-66; quiz 667. doi: 10.1038/ajg.2012.49. Epub 2012 Apr 10. PMID: 22488077.
- Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010 Feb;25(2):252-8. doi: 10.1111/j.1440-1746.2009.06149.x. PMID: 20136989.
- Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012 May;107(5):657-66; quiz 667. doi: 10.1038/ajg.2012.49. Epub 2012 Apr 10. PMID: 22488077.
- Whalen KA, McCullough ML, Flanders WD, Hartman TJ, Judd S, Bostick RM. Paleolithic and Mediterranean Diet Pattern Scores Are Inversely Associated with Biomarkers of Inflammation and Oxidative Balance in Adults. J Nutr. 2016 Jun;146(6):1217-26. doi: 10.3945/jn.115.224048. Epub 2016 Apr 20. PMID: 27099230; PMCID: PMC4877627.
- Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J. 2014 Jan 16;13:5. doi: 10.1186/1475-2891-13-5. PMID: 24428901; PMCID: PMC3896778.
- Konijeti GG, Kim N, Lewis JD, Groven S, Chandrasekaran A, Grandhe S, Diamant C, Singh E, Oliveira G, Wang X, Molparia B, Torkamani A. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 Nov;23(11):2054-2060. doi: 10.1097/MIB.0000000000001221. PMID: 28858071; PMCID: PMC5647120.
- Masharani U, Sherchan P, Schloetter M, Stratford S, Xiao A, Sebastian A, Nolte Kennedy M, Frassetto L. Metabolic and physiologic effects from consuming a hunter-gatherer (Paleolithic)-type diet in type 2 diabetes. Eur J Clin Nutr. 2015 Aug;69(8):944-8. doi: 10.1038/ejcn.2015.39. Epub 2015 Apr 1. PMID: 25828624.
- Jönsson T, Granfeldt Y, Ahrén B, Branell UC, Pålsson G, Hansson A, Söderström M, Lindeberg S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009 Jul 16;8:35. doi: 10.1186/1475-2840-8-35. PMID: 19604407; PMCID: PMC2724493.
- Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. Am J Clin Nutr. 2015 Oct;102(4):922-32. doi: 10.3945/ajcn.115.113613. Epub 2015 Aug 12. PMID: 26269362; PMCID: PMC4588744.
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007 Aug 16;2(2):119-29. doi: 10.1016/j.chom.2007.06.010. PMID: 18005726.
- Nistal E, Caminero A, Herrán AR, Arias L, Vivas S, de Morales JM, Calleja S, de Miera LE, Arroyo P, Casqueiro J. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: effect of age, gluten diet, and disease. Inflamm Bowel Dis. 2012 Apr;18(4):649-56. doi: 10.1002/ibd.21830. Epub 2011 Aug 8. PMID: 21826768.
- Chen B, Kim JJ, Zhang Y, Du L, Dai N. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol. 2018 Jul;53(7):807-818. doi: 10.1007/s00535-018-1476-9. Epub 2018 May 14. PMID: 29761234.
- Schmulson M, Bielsa MV, Carmona-Sánchez R, Hernández A, López-Colombo A, López Vidal Y, Peláez-Luna M, Remes-Troche JM, Tamayo JL, Valdovinos MA. Microbiota, gastrointestinal infections, low-grade inflammation, and antibiotic therapy in irritable bowel syndrome: an evidence-based review. Rev Gastroenterol Mex. 2014 Apr-Jun;79(2):96-134. English, Spanish. doi: 10.1016/j.rgmx.2014.01.004. Epub 2014 May 23. PMID: 24857420.
- Takakura W, Pimentel M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome – An Update. Front Psychiatry. 2020 Jul 10;11:664. doi: 10.3389/fpsyt.2020.00664. PMID: 32754068; PMCID: PMC7366247.
- Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017 Apr;49(4):331-337. doi: 10.1016/j.dld.2017.01.142. Epub 2017 Jan 21. PMID: 28179092.
- Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr. 2000 Jul;31(1):8-15. doi: 10.1097/00005176-200007000-00005. PMID: 10896064.
- Rajendran N, Kumar D. Role of diet in the management of inflammatory bowel disease. World J Gastroenterol. 2010 Mar 28;16(12):1442-8. doi: 10.3748/wjg.v16.i12.1442. PMID: 20333783; PMCID: PMC2846248.
- Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, Russo PM, Cucchiara S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006 Jun;4(6):744-53. doi: 10.1016/j.cgh.2006.03.010. Epub 2006 May 6. PMID: 16682258.
- Knight C, El-Matary W, Spray C, Sandhu BK. Long-term outcome of nutritional therapy in paediatric Crohn’s disease. Clin Nutr. 2005 Oct;24(5):775-9. doi: 10.1016/j.clnu.2005.03.005. PMID: 15904998.
- Day AS, Whitten KE, Sidler M, Lemberg DA. Systematic review: nutritional therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2008 Feb 15;27(4):293-307. doi: 10.1111/j.1365-2036.2007.03578.x. Epub 2007 Nov 27. PMID: 18045244.
- Verma S, Brown S, Kirkwood B, Giaffer MH. Polymeric versus elemental diet as primary treatment in active Crohn’s disease: a randomized, double-blind trial. Am J Gastroenterol. 2000 Mar;95(3):735-9. doi: 10.1111/j.1572-0241.2000.01527.x. PMID: 10710067.
- Hiwatashi N. Enteral nutrition for Crohn’s disease in Japan. Dis Colon Rectum. 1997 Oct;40(10 Suppl):S48-53. doi: 10.1007/BF02062020. PMID: 9378012.
- Pimentel M, Constantino T, Kong Y, Bajwa M, Rezaei A, Park S. A 14-day elemental diet is highly effective in normalizing the lactulose breath test. Dig Dis Sci. 2004 Jan;49(1):73-7. doi: 10.1023/b:ddas.0000011605.43979.e1. PMID: 14992438.
- Olaussen RW, Løvik A, Tollefsen S, Andresen PA, Vatn MH, De Lange T, Bratlie J, Brandtzaeg P, Farstad IN, Lundin KE. Effect of elemental diet on mucosal immunopathology and clinical symptoms in type 1 refractory celiac disease. Clin Gastroenterol Hepatol. 2005 Sep;3(9):875-85. doi: 10.1016/s1542-3565(05)00295-8. PMID: 16234025.
- Lackner S, Malcher V, Enko D, Mangge H, Holasek SJ, Schnedl WJ. Histamine-reduced diet and increase of serum diamine oxidase correlating to diet compliance in histamine intolerance. Eur J Clin Nutr. 2019 Jan;73(1):102-104. doi: 10.1038/s41430-018-0260-5. Epub 2018 Jul 18. PMID: 30022117.
- Schink M, Konturek PC, Tietz E, Dieterich W, Pinzer TC, Wirtz S, Neurath MF, Zopf Y. Microbial patterns in patients with histamine intolerance. J Physiol Pharmacol. 2018 Aug;69(4). doi: 10.26402/jpp.2018.4.09. Epub 2018 Dec 9. PMID: 30552302.
- Chen B, Kim JJ, Zhang Y, Du L, Dai N. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol. 2018 Jul;53(7):807-818. doi: 10.1007/s00535-018-1476-9. Epub 2018 May 14. PMID: 29761234.
- Schmulson M, Bielsa MV, Carmona-Sánchez R, Hernández A, López-Colombo A, López Vidal Y, Peláez-Luna M, Remes-Troche JM, Tamayo JL, Valdovinos MA. Microbiota, gastrointestinal infections, low-grade inflammation, and antibiotic therapy in irritable bowel syndrome: an evidence-based review. Rev Gastroenterol Mex. 2014 Apr-Jun;79(2):96-134. English, Spanish. doi: 10.1016/j.rgmx.2014.01.004. Epub 2014 May 23. PMID: 24857420.
- Takakura W, Pimentel M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome – An Update. Front Psychiatry. 2020 Jul 10;11:664. doi: 10.3389/fpsyt.2020.00664. PMID: 32754068; PMCID: PMC7366247.
- Chandrasekaran A, Groven S, Lewis JD, Levy SS, Diamant C, Singh E, Konijeti GG. An Autoimmune Protocol Diet Improves Patient-Reported Quality of Life in Inflammatory Bowel Disease. Crohns Colitis 360. 2019 Oct;1(3):otz019. doi: 10.1093/crocol/otz019. Epub 2019 Aug 7. PMID: 31832627; PMCID: PMC6892563.
- Konijeti GG, Kim N, Lewis JD, Groven S, Chandrasekaran A, Grandhe S, Diamant C, Singh E, Oliveira G, Wang X, Molparia B, Torkamani A. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 Nov;23(11):2054-2060. doi: 10.1097/MIB.0000000000001221. PMID: 28858071; PMCID: PMC5647120.
- Chandrasekaran A, Molparia B, Akhtar E, Wang X, Lewis JD, Chang JT, Oliveira G, Torkamani A, Konijeti GG. The Autoimmune Protocol Diet Modifies Intestinal RNA Expression in Inflammatory Bowel Disease. Crohns Colitis 360. 2019 Oct;1(3):otz016. doi: 10.1093/crocol/otz016. Epub 2019 Jul 12. PMID: 32309803; PMCID: PMC7147823.
- McFarlin BK, Henning AL, Bowman EM, Gary MA, Carbajal KM. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J Gastrointest Pathophysiol. 2017 Aug 15;8(3):117-126. doi: 10.4291/wjgp.v8.i3.117. PMID: 28868181; PMCID: PMC5561432.
- Lamprecht M, Bogner S, Schippinger G, Steinbauer K, Fankhauser F, Hallstroem S, Schuetz B, Greilberger JF. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012 Sep 20;9(1):45. doi: 10.1186/1550-2783-9-45. PMID: 22992437; PMCID: PMC3465223.
- Mujagic Z, de Vos P, Boekschoten MV, Govers C, Pieters HH, de Wit NJ, Bron PA, Masclee AA, Troost FJ. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci Rep. 2017 Jan 3;7:40128. doi: 10.1038/srep40128. PMID: 28045137; PMCID: PMC5206730.
- Bonfrate L, Di Palo DM, Celano G, Albert A, Vitellio P, De Angelis M, Gobbetti M, Portincasa P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur J Clin Invest. 2020 Mar;50(3):e13201. doi: 10.1111/eci.13201. Epub 2020 Feb 12. PMID: 31960952.
- Francavilla R, Miniello V, Magistà AM, De Canio A, Bucci N, Gagliardi F, Lionetti E, Castellaneta S, Polimeno L, Peccarisi L, Indrio F, Cavallo L. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010 Dec;126(6):e1445-52. doi: 10.1542/peds.2010-0467. Epub 2010 Nov 15. PMID: 21078735.
- Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM. Probiotic Supplementation in Patients with Alzheimer’s Dementia – An Explorative Intervention Study. Curr Alzheimer Res. 2018;15(12):1106-1113. doi: 10.2174/1389200219666180813144834. PMID: 30101706; PMCID: PMC6340155.
- McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008 May 7;14(17):2650-61. doi: 10.3748/wjg.14.2650. PMID: 18461650; PMCID: PMC2709042.
- Martoni CJ, Srivastava S, Leyer GJ. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients. 2020 Jan 30;12(2):363. doi: 10.3390/nu12020363. PMID: 32019158; PMCID: PMC7071206.
- Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care. 2011 Nov;14(6):581-7. doi: 10.1097/MCO.0b013e32834b8082. PMID: 21892075.
- Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014 Oct;109(10):1547-61; quiz 1546, 1562. doi: 10.1038/ajg.2014.202. Epub 2014 Jul 29. PMID: 25070051.
- Ibarra A, Latreille-Barbier M, Donazzolo Y, Pelletier X, Ouwehand AC. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: A double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes. 2018;9(3):236-251. doi: 10.1080/19490976.2017.1412908. Epub 2018 Feb 8. PMID: 29227175; PMCID: PMC6219592.
- Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014 Oct;109(10):1547-61; quiz 1546, 1562. doi: 10.1038/ajg.2014.202. Epub 2014 Jul 29. PMID: 25070051.
- Rogha M, Esfahani MZ, Zargarzadeh AH. The efficacy of a synbiotic containing Bacillus Coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterol Hepatol Bed Bench. 2014 Summer;7(3):156-63. PMID: 25120896; PMCID: PMC4129566.
- Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, Pande A, Majeed S, Karri SK. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: a double blind randomized placebo controlled pilot clinical study. Nutr J. 2016 Feb 27;15:21. doi: 10.1186/s12937-016-0140-6. PMID: 26922379; PMCID: PMC4769834.
- Khalighi AR, Khalighi MR, Behdani R, Jamali J, Khosravi A, Kouhestani Sh, Radmanesh H, Esmaeelzadeh S, Khalighi N. Evaluating the efficacy of probiotic on treatment in patients with small intestinal bacterial overgrowth (SIBO)–a pilot study. Indian J Med Res. 2014 Nov;140(5):604-8. PMID: 25579140; PMCID: PMC4311312.
- Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM. Probiotic Supplementation in Patients with Alzheimer’s Dementia – An Explorative Intervention Study. Curr Alzheimer Res. 2018;15(12):1106-1113. doi: 10.2174/1389200219666180813144834. PMID: 30101706; PMCID: PMC6340155.
- Toribio-Mateas M. Harnessing the Power of Microbiome Assessment Tools as Part of Neuroprotective Nutrition and Lifestyle Medicine Interventions. Microorganisms. 2018 Apr 25;6(2):35. doi: 10.3390/microorganisms6020035. PMID: 29693607; PMCID: PMC6027349.
- Wang F, Feng J, Chen P, Liu X, Ma M, Zhou R, Chang Y, Liu J, Li J, Zhao Q. Probiotics in Helicobacter pylori eradication therapy: Systematic review and network meta-analysis. Clin Res Hepatol Gastroenterol. 2017 Sep;41(4):466-475. doi: 10.1016/j.clinre.2017.04.004. Epub 2017 May 25. PMID: 28552432.
- García-Collinot G, Madrigal-Santillán EO, Martínez-Bencomo MA, Carranza-Muleiro RA, Jara LJ, Vera-Lastra O, Montes-Cortes DH, Medina G, Cruz-Domínguez MP. Effectiveness of Saccharomyces boulardii and Metronidazole for Small Intestinal Bacterial Overgrowth in Systemic Sclerosis. Dig Dis Sci. 2020 Apr;65(4):1134-1143. doi: 10.1007/s10620-019-05830-0. Epub 2019 Sep 23. PMID: 31549334.
- Greco A, Caviglia GP, Brignolo P, Ribaldone DG, Reggiani S, Sguazzini C, Smedile A, Pellicano R, Resegotti A, Astegiano M, Bresso F. Glucose breath test and Crohn’s disease: Diagnosis of small intestinal bacterial overgrowth and evaluation of therapeutic response. Scand J Gastroenterol. 2015;50(11):1376-81. doi: 10.3109/00365521.2015.1050691. Epub 2015 May 19. PMID: 25990116.
- American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009 Jan;104 Suppl 1:S1-35. doi: 10.1038/ajg.2008.122. PMID: 19521341.
- Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014 Oct;109(10):1547-61; quiz 1546, 1562. doi: 10.1038/ajg.2014.202. Epub 2014 Jul 29. PMID: 25070051.
- El-Salhy M, Ystad SO, Mazzawi T, Gundersen D. Dietary fiber in irritable bowel syndrome (Review). Int J Mol Med. 2017 Sep;40(3):607-613. doi: 10.3892/ijmm.2017.3072. Epub 2017 Jul 19. PMID: 28731144; PMCID: PMC5548066.
- EASTOE JE. The amino acid composition of mammalian collagen and gelatin. Biochem J. 1955 Dec;61(4):589-600. doi: 10.1042/bj0610589. PMID: 13276342; PMCID: PMC1215839.
- De Luca C, Mikhal’chik EV, Suprun MV, Papacharalambous M, Truhanov AI, Korkina LG. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxid Med Cell Longev. 2016;2016:4389410. doi: 10.1155/2016/4389410. Epub 2016 Jan 19. PMID: 26904164; PMCID: PMC4745978.
- Kim DU, Chung HC, Choi J, Sakai Y, Lee BY. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2018 Jun 26;10(7):826. doi: 10.3390/nu10070826. PMID: 29949889; PMCID: PMC6073484.
- Asserin, J., Lati, E., Shioya, T. and Prawitt, J. (2015), The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo‐controlled clinical trials. J Cosmet Dermatol, 14: 291-301. https://doi.org/10.1111/jocd.12174
- Chen Q, Chen O, Martins IM, Hou H, Zhao X, Blumberg JB, Li B. Collagen peptides ameliorate intestinal epithelial barrier dysfunction in immunostimulatory Caco-2 cell monolayers via enhancing tight junctions. Food Funct. 2017 Mar 22;8(3):1144-1151. doi: 10.1039/c6fo01347c. PMID: 28174772.
- Lin M, Zhang B, Yu C, Li J, Zhang L, Sun H, Gao F, Zhou G. L-Glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PLoS One. 2014 Nov 4;9(11):e111950. doi: 10.1371/journal.pone.0111950. PMID: 25368996; PMCID: PMC4219819.
- Tariq M, Al Moutaery AR. Studies on the antisecretory, gastric anti-ulcer and cytoprotective properties of glycine. Res Commun Mol Pathol Pharmacol. 1997 Aug;97(2):185-98. PMID: 9344231.
- Rezac S, Kok CR, Heermann M, Hutkins R. Fermented Foods as a Dietary Source of Live Organisms. Front Microbiol. 2018 Aug 24;9:1785. doi: 10.3389/fmicb.2018.01785. PMID: 30197628; PMCID: PMC6117398.
- LAYE, I., KARLESKIND, D. and MORR, C. (1993), Chemical, Microbiological and Sensory Properties of Plain Nonfat Yogurt. Journal of Food Science, 58: 991-995. https://doi.org/10.1111/j.1365-2621.1993.tb06096.x
- https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2621.1993.tb06096.x
- https://www.olivemypickle.com/blogs/news/probiotics-in-our-pickles-microbiology-lab-verified
- Money ME, Walkowiak J, Virgilio C, Talley NJ. Pilot study: a randomised, double blind, placebo controlled trial of pancrealipase for the treatment of postprandial irritable bowel syndrome-diarrhoea. Frontline Gastroenterol. 2011 Jan;2(1):48-56. doi: 10.1136/fg.2010.002253. Epub 2010 Nov 3. PMID: 22095308; PMCID: PMC3009417.
- Spagnuolo R, Cosco C, Mancina RM, Ruggiero G, Garieri P, Cosco V, Doldo P. Beta-glucan, inositol and digestive enzymes improve quality of life of patients with inflammatory bowel disease and irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2017 Jun;21(2 Suppl):102-107. PMID: 28724171.
- Ciacci C, Franceschi F, Purchiaroni F, Capone P, Buccelletti F, Iacomini P, Ranaudo A, Andreozzi P, Tondi P, Gentiloni Silveri N, Gasbarrini A, Gasbarrini G. Effect of beta-glucan, inositol and digestive enzymes in GI symptoms of patients with IBS. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):637-43. PMID: 21796867.
- Weir I, Shu Q, Wei N, Wei C, Zhu Y. Efficacy of actinidin-containing kiwifruit extract Zyactinase on constipation: a randomised double-blinded placebo-controlled clinical trial. Asia Pac J Clin Nutr. 2018;27(3):564-571. doi: 10.6133/apjcn.122017.03. PMID: 29737803.
- Sundin, O.H., Mendoza-Ladd, A., Zeng, M. et al. The human jejunum has an endogenous microbiota that differs from those in the oral cavity and colon. BMC Microbiol 17, 160 (2017). https://doi.org/10.1186/s12866-017-1059-6
- Marshall BJ (1994) Helicobacter pylori. Am J Gastroenterol 89: 116-118.
- Miceli E, Lenti MV, Padula D, Luinetti O, Vattiato C, Monti CM, Di Stefano M, Corazza GR. Common features of patients with autoimmune atrophic gastritis. Clin Gastroenterol Hepatol. 2012 Jul;10(7):812-4. doi: 10.1016/j.cgh.2012.02.018. Epub 2012 Mar 2. PMID: 22387252.
- De Block CE, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab. 2008 Feb;93(2):363-71. doi: 10.1210/jc.2007-2134. Epub 2007 Nov 20. PMID: 18029461.
- De Block CE, De Leeuw IH, Bogers JJ, Pelckmans PA, Ieven MM, Van Marck EA, Van Acker KL, Van Gaal LF. Autoimmune gastropathy in type 1 diabetic patients with parietal cell antibodies: histological and clinical findings. Diabetes Care. 2003 Jan;26(1):82-8. doi: 10.2337/diacare.26.1.82. PMID: 12502662.
- Lahner E, Annibale B. Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol. 2009 Nov 7;15(41):5121-8. doi: 10.3748/wjg.15.5121. PMID: 19891010; PMCID: PMC2773890.
- Sterzl I, Hrdá P, Matucha P, Cerovská J, Zamrazil V. Anti-Helicobacter Pylori, anti-thyroid peroxidase, anti-thyroglobulin and anti-gastric parietal cells antibodies in Czech population. Physiol Res. 2008;57 Suppl 1:S135-41. Epub 2008 Feb 13. PMID: 18271683.
- Gillberg R, Kastrup W, Mobacken H, Stockbrügger R, Ahren C. Gastric morphology and function in dermatitis herpetiformis and in coeliac disease. Scand J Gastroenterol. 1985 Mar;20(2):133-40. doi: 10.3109/00365528509089645. PMID: 3992169.
- Kang MS, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. [Bamboo joint-like appearance of stomach in Korean patients with Crohn’s disease]. Korean J Gastroenterol. 2006 Dec;48(6):395-400. Korean. PMID: 17189922.
- Akiyama, T., Kishimoto, S. & Miyaji, K. Gastric acid secretion, serum gastrin and parietal cell histology in hyperthyroidism. Gastroenterol Jpn 17, 42–49 (1982). https://doi.org/10.1007/BF02774760
- Sugaya T, Sakai H, Sugiyama T, Imai K. [Atrophic gastritis in Sjögren’s syndrome]. Nihon Rinsho. 1995 Oct;53(10):2540-4. Japanese. PMID: 8531370.
- Andrea L Betesh, Carol A Santa Ana, Jason A Cole, John S Fordtran, Is achlorhydria a cause of iron deficiency anemia?, The American Journal of Clinical Nutrition, Volume 102, Issue 1, July 2015, Pages 9–19, https://doi.org/10.3945/ajcn.114.097394
- Yago MR, Frymoyer AR, Smelick GS, Frassetto LA, Budha NR, Dresser MJ, Ware JA, Benet LZ. Gastric reacidification with betaine HCl in healthy volunteers with rabeprazole-induced hypochlorhydria. Mol Pharm. 2013 Nov 4;10(11):4032-7. doi: 10.1021/mp4003738. Epub 2013 Sep 10. PMID: 23980906; PMCID: PMC3946491.
- Yago MR, Frymoyer A, Benet LZ, Smelick GS, Frassetto LA, Ding X, Dean B, Salphati L, Budha N, Jin JY, Dresser MJ, Ware JA. The use of betaine HCl to enhance dasatinib absorption in healthy volunteers with rabeprazole-induced hypochlorhydria. AAPS J. 2014 Nov;16(6):1358-65. doi: 10.1208/s12248-014-9673-9. Epub 2014 Oct 2. PMID: 25274610; PMCID: PMC4389759.
- Hall JC, Heel K, McCauley R. Glutamine. Br J Surg. 1996 Mar;83(3):305-12. doi: 10.1002/bjs.1800830306. PMID: 8665180.
- Rao R, Samak G. Role of Glutamine in Protection of Intestinal Epithelial Tight Junctions. J Epithel Biol Pharmacol. 2012 Jan;5(Suppl 1-M7):47-54. doi: 10.2174/1875044301205010047. PMID: 25810794; PMCID: PMC4369670.
- Shu XL, Yu TT, Kang K, Zhao J. Effects of glutamine on markers of intestinal inflammatory response and mucosal permeability in abdominal surgery patients: A meta-analysis. Exp Ther Med. 2016 Dec;12(6):3499-3506. doi: 10.3892/etm.2016.3799. Epub 2016 Oct 12. PMID: 28105083; PMCID: PMC5228558.
- Kim MH, Kim H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int J Mol Sci. 2017 May 12;18(5):1051. doi: 10.3390/ijms18051051. PMID: 28498331; PMCID: PMC5454963.




Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!