Practitioner Research Review – August 2019
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
In Today’s Issue
Research
- Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders
- Neurological Dysfunction in Coeliac Disease and Non-Coeliac Gluten Sensitivity
- Rapid-fire Research – ultra concise summaries of noteworthy studies
- Urinary Iodine Concentration Is Inversely Associated With Thyroglobulin Antibodies.
- Selenium Supplementation In Patients With Subclinical Hypothyroidism Affected By Autoimmune Thyroiditis: Results Of The SETI Study.
- Epithelial Response To Butyrate Diminished In Active IBD
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however, the research studies are a collection of the most clinically meaningful research that has been published recently.
Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders
Nat Commun. 2019 May 1;10(1):2012. doi: 10.1038/s41467-019-09964-7.
Study purpose
- Correlate SIBO test from duodenal aspirates to duodenal biopsy, and stool tests with patients’ symptoms in those with GI symptoms
Intervention:
- 126 patients with GI symptoms was assessed with duodenal aspirates for SIBO.
Main Results:
- Note: this paper could easily be misread by a SIBO detractor, as I will point out below.
- Only 50% of symptomatic patients tested positive for SIBO. All healthy controls were negative for SIBO.
- Of the 126 patients, 66 (52%) tested positive for SIBO, whereas 60 patients (48%) tested negative for SIBO
- The duodenal aspirate microbiome is altered in symptomatic patients.
- The small intestinal microbial communities from symptomatic patients were characterized by significantly lower phylogenetic alpha diversity, richness, and evenness
- Beware misleading statements….. Authors statement:
- “A subset of symptomatic patients have small intestinal microbial dysbiosis”.
- What the authors don’t make apparent
- 3% of healthy controls had ‘dysbiosis’ and 29% of symptomatic were found to be dysbiotic.
- This classification scheme identified 37/38 (97%) healthy volunteers as “healthy-like”, whereas 89 (71%) symptomatic patient communities were found to be healthy-like and 37 (29%) were found to be dysbiotic (based on distance from healthy
- Why this matters? If you compare the numbers, the SIBO breath test from aspirates performed better than the duodenal biopsy dysbiosis assessment.
- SIBO testing from aspirates doesn’t correlate with duodenal biopsy dysbiosis assessment.
- SIBO does not correlate with small intestinal microbial dysbiosis. We compared the microbial composition of symptomatic patients with and without SIBO and found that SIBO does not correlate with small intestinal dysbiosis (p = 0.33).
- A subset of healthy individuals eating high-fiber diets has SIBO. YES. This is important and is another example of why we should NOT look at lab results in isolation. There are SIBO positive cases who are healthy and do not require treatment.
- Despite being asymptomatic, 8/16 (50%) subjects on a baseline high-fiber diet tested positive for SIBO by the standard culture criteria described above.
Limitations:
- R’s notes: duodenal aspirates are not perfect because only measures the first section of the small intestine. It is possible that cases were missed due to ‘distal SIBO’. However, if they would have performed SIBO lactulose breath tests, we could have a quite different abundance picture of the small intestine microbes.
Interesting Notes:
- We don’t know as much as we think. Many ‘gut gurus’ have proclaimed how harmful Prevotella is, however things are not that simple in the complex ecosystem of the gut. Symptomatic patients have less of the ‘harmful’ Prevotella…..
- We found that small intestinal microbial composition was significantly altered in patients with GI symptoms, including significant decreases in members of genus Prevotella
Clinical Takeaways:
- SIBO breath testing is not perfect, some healthy cases may test positive for SIBO
- Some SIBO negative cases may have other imbalances, namely SI dysbiosis, and thus require treatment
- What to tell your patients:
- Mention the above, and
- GI testing is imperfect. We can use testing but it is only one part of the equation is improving your gut health. Your history, symptom, and response to treatment are all equally if not more important.
Dr. Ruscio Comments
I read this paper with great interest at first but later became disappointed. It does provide some interesting and useful insights, but the narrative and the data don’t appear to match. This why it’s so important to read beyond the abstract. The data here are interesting and suggest dysbiosis can be present beyond and independent of SIBO. But the sense you obtain from the abstract is that this paper is showing SIBO to be irrelevant. This might not have been intentional and the authors may have simply been excited by a novel finding. I try to always summarize the findings, however novel, back to a narrative that is grounded in a clinical context. Sometimes the excitement of a novel academic finding takes a clinician away from the clinical context ie what do I do differently in the clinic because of this.
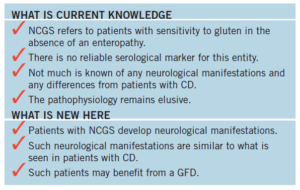
Neurological Dysfunction in Coeliac Disease and Non-Coeliac Gluten Sensitivity
Am J Gastroenterol. 2016 Apr;111(4):561-7. doi: 10.1038/ajg.2015.434. Epub 2016 Feb 2.
Study purpose
- Investigate the clinical and immunological characteristics of patients presenting with neurological manifestations with CD and those with NCGS
Intervention:
- We compared clinical, neurophysiological, and imaging data of 562 patients with CD and NCGS presenting with neurological dysfunction assessed and followed up regularly over a period of 20 years.
Main Results:
- All patients presented with neurological dysfunction and had circulating anti-gliadin antibodies.
- Roughly 60% of those with NCGS and neurological manifestation in reaction to gluten did not have enteropathy (ie had normal intestinal biopsies).
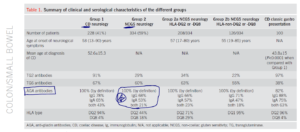
- Out of 562 patients, 228 (41%) had evidence of enteropathy (Group 1, CD) and 334 (59%) did not (Group 2, NCGS).
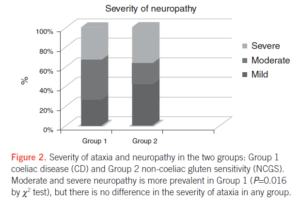
- The most common neurological manifestations were cerebellar ataxia, peripheral neuropathy, and encephalopathy.
- Reminder: Encephalopathy can present a very broad spectrum of symptoms that range from mild, such as some memory loss or subtle personality changes, to severe, such as dementia, seizures, coma, or death.
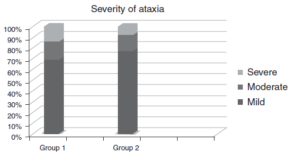
- The severity of ataxia did not differ between the two groups.
- All patients from both groups responded to a gluten-free diet.
- Transglutaminase antibodies were only found in 29% of NCGS (group 2)
- Anti-tissue transglutaminase (TG2) antibodies were found in 91% of patients in Group 1 and in 29% of patients in Group 2.
- This indicates, as expected, there is a high degree of correlation between enteropathy and circulating anti-TG2 antibodies.
- Genetic testing did not help identify those with NCGS and the neurological manifestation
- Comparison between those patients in Group 2 with HLA-DQ2/DQ8 and those without as well as those with positive TG2 compared with those with negative TG2 antibodies identified no differences within these subgroups.
- Transglutaminase 6 antibodies (neurological tissue) were similar in both groups (67% celiac and 60% NCGS)
- Serological positivity for TG6 antibodies was similar in the two groups (67 and 60%).
Additional Results:
- HLA typing was positive in 98% of celiac pts, and in 62% of NCGS patients.
- AGA antibodies
- Celiac: AGA IgG was positive in 78% of patients
- Celiac: AGA IgA was positive in 65% of patients
- NCGS: AGA IgG was positive in 68% of patients
- NCGS: AGA IgA was positive in 53% of patients
- So, serum AGA IgG appears a good marker for NCGS, unfortunately, LabCorp does not offer this as a stand-alone marker.
- AGA can provide an indication that further investigation is warranted, although not diagnostic by itself.
- AGA can provide an indication that further investigation is warranted, although not diagnostic by itself.
- The severity of clinical manifestation was similar between celiac and NCGS
Limitations:
- This was a retrospective analysis
Authors Conclusion:
- This is a retrospective review of probably the largest cohort of patients presenting with neurological manifestations of GRD suggests that there are no clear distinguishing neurological features between those patients with CD and those with NCGS. Irrespective of these differences, the neurological manifestations in both groups were equally responsive to a GFD.
- An important finding in this study is that patients with NCGS can present with neurological dysfunction in an identical manner to those patients with CD, suggesting similar immunological processes being responsible at least for the neural damage. This is also supported by the similar prevalence of TG6 antibodies in the two groups
- Currently, the best approach would be to include all serological testing (TG2, TG6, anti-endomysium antibodies, AGA) for patients suspected of having GRD.
Interesting Notes:
- Celiac may manifest solely extra-intestinally
- Classic presentations of CD such as abdominal bloating, weight loss, diarrhea, anemia, and malabsorption are no longer the norm and patients can present with minimal or no gastrointestinal symptoms and diverse extraintestinal manifestations affecting other organs such as the skin and the nervous system
- TG2 (transglutaminase 2) antibodies may not be positive in NCGS because those antibodies indicate intestinal damage, whereas some NCGS may have no frank intestinal damage and only see lesioning at extra-intestinal locations like brain, skin, etc…
- Although some markers such as anti-tissue transglutaminase (TG2) autoantibodies detected as anti-endomysium antibodies or by ELISA (anti-TG2 antibodies) are sensitive and specific in diagnosing CD, such antibodies will usually be absent in patients with NCGS. This may reflect the absence of detectable levels of circulating antibodies in some cases where the immune response is distant from the gut (e.g., cerebellum in gluten ataxia).
- Anti-gliadin IgG may be the best marker for NCGS, being positive in roughly 50% of cases
- Anti-gliadin antibodies (AGA) may be an indicator of NCGS as up to 50% of such patients presenting to gastroenterologists have detectable circulating levels, primarily of IgG AGA [8].
- Other tests may help assess autoimmune damage at distal sites. Dr. R’s note: my caution with these tests is lack of validation and being limited to one lab/group thus not been verified by others – elimination/reintroduction is likely the best method at present.
- More specific markers, i.e., antibodies directed at autoantigens likely to be responsible for extraintestinal manifestations, have been identified but are not yet in general use
- Antibodies against TG3, an epidermal TG, have been found in patients with DH, whereas antibodies against TG6, a brain expressed TG, have been found in patients with gluten ataxia (9,10). However, the extent of overlap between NCGS, DH, and gluten ataxia is unclear at present and awaits the development of validated diagnostic approaches. [9,10]
- HLA gene testing is not clinically helpful for those with NCGS
- HLA type, however, cannot be interpreted in isolation as DQ2 is found in up to 25% of the healthy population, of which only a fraction will ever develop GRDs (gluten-related disorders).
- Furthermore, while there is over-representation of DQ2 in patients with NCGS, a significant minority do not have the HLA-DQ2 or -DQ8 and yet appear to respond to a GFD.
- In those with celiac, sequelae may start in the gut and then later develop in the brain. Those with only neurological manifestations are diagnosed 10 years later on average, indicating that clinicians are not privy to the neurological manifestations of celiac.
- The presence of TG6 antibodies in 38% of patients with newly diagnosed CD presenting with the classic gastrointestinal symptoms to a gastroenterologist may suggest that these patients are susceptible to future development of neurological dysfunction if they continue to consume gluten.
- This is also supported by the fact that patients with CD presenting with neurological problems are likely to be diagnosed with CD significantly later (mean age 52.6±15.3 years) when compared with those presenting with gastrointestinal symptoms (mean age 43.8±15 years).
- It could be argued that the presence of gastrointestinal symptoms offers a therapeutic advantage to these patients as it increases the likelihood of them being diagnosed and treated early, as opposed to the neurology patients who on average are diagnosed 10 years later.
Clinical Takeaways:
- Gluten intolerance, whether celiac or NCGS, could solely manifest neurologically
- The most common manifestation is ataxia, neuropathy, and encephalopathy. The presentation can be subtle and may elude clinicians who are not on the lookout.
- What to tell your patients:
- Gluten reactivity may solely manifest neurologically. We will first start with an elimination diet AND improving your digestive health. This will reduce symptoms and food reactions, thus making it the appropriate time for a gluten reintroduction. During your reintroduction, we will be on the lookout for any symptoms that appear shortly after gluten reintroduction. Also, remember that not everyone has a problem with gluten and thus we will also want to be cautious to not create the expectation of a reaction.
Dr. Ruscio Comments
While we as clinicians must be careful not to bias our patients into thinking they have a problem with gluten when they do not, we also must help patients identify this food trigger.
Reduce cofounding variables by improving GI health, then this is the right time for a non-biased and objective reintroduction.
Rapid-Fire Research: Ultra-Concise Summaries of Noteworthy Studies
Urinary Iodine Concentration Is Inversely Associated With Thyroglobulin Antibodies
Endocr Pract. 2019 May;25(5):454-460. doi: 10.4158/EP-2018-0252. Epub 2019 Jan 18.
- Summary: Low iodine increases TGabs
- Conclusion: Low UIC is an independent risk factor for positive TgAb in individuals living in iodine-sufficient areas.
- Notes: while a high intake of iodine can be problematic for thyroid, inadequate intake may also lead to suboptimal thyroid function. See my two-part series on iodine and thyroid here.
Selenium Supplementation In Patients With Subclinical Hypothyroidism Affected By Autoimmune Thyroiditis: Results Of The SETI Study
Endocrinol Diabetes Nutr. 2019 Jun 10. pii: S2530-0164(19)30116-8. doi: 10.1016/j.endinu.2019.03.018.
- Summary:
- Selenium normalized subclinical hypothyroid in roughly 50% of cases, with no impact on TPO antibodies.
- Setup:
- Patients with subclinical hypothyroidism due to Hashimoto thyroiditis were prospectively enrolled in the SETI study.
- They received 83 mcg of selenomethionine/day orally in a soft gel capsule for 4 months with water after a meal.
- No further treatment was given.
- Results:
- 50 patients
- At the end of the study, euthyroidism was restored in 22/45 (48.9%) participants (responders),
- 23 patients remained hypothyroid (non-responders).
- There were no significant changes in TPOAb or iodine levels from baseline to the end of the study in both responders and non-responders.
- TSH levels were re-tested six months after selenomethionine withdrawal: 3% of responding patients remained euthyroid, while only 14.2% of non-responders became euthyroid.
- CONCLUSIONS:
- The SETI study shows that short-course supplementation with selenomethionine is associated to a normalization of serum TSH levels which is maintained 6 months after selenium withdrawal in 50% of patients with subclinical hypothyroidism due to chronic autoimmune thyroiditis.
- This TSH-lowering effect of selenium supplementation is unlikely to be related to changes in humoral markers of autoimmunity
Epithelial Response To Butyrate Diminished In Active IBD
Inflamm Bowel Dis. 2019 Jun 18. pii: izz119. doi: 10.1093/ibd/izz119. [Epub ahead of print]
- Summary:
- Inflammation may reduce response to butyrate, so it may be better to wait on supplemental butyrate or fiber/prebiotic supplementation until IBD patients are in remission. This is common sense, but know we are seeing mechanism which underlies this recommendation.
- Conclusions:
- “We provide evidence that the response to butyrate is not intrinsically altered in IBD patients. However, TNFα renders the epithelium less responsive to this metabolite, defeating the purpose of butyrate supplementation during active inflammation.”
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next practitioner question of the month.

Like what you’re reading?
Please share this with a colleague and help us improve functional medicine
Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!