Practitioner Research Review – December 2018
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
In Today’s Issue
Research
- Mechanism, Clinical Studies and Distinctive Nutritional Requirement of Oral Immunoglobulins in IBS-D and SIBO
- The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study
- Fluctuation of zonulin levels in blood vs stability of antibodies
- Rapid-Fire Research – Ultra-concise summaries of noteworthy studies
- A low FODMAP gluten-fee diet improves functional gastrointestinal disorders and Overall Mental Health of Celiac Disease Patients: A Randomized Controlled Trial
- Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome
- Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: A systematic review and meta-analysis
- Thyroxine and triiodothyronine content in commercially available thyroid health supplements
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however, the research studies are a collection of the most clinically meaningful research that has been published recently.
Mechanism, Clinical Studies and Distinctive Nutritional Requirement of Oral Immunoglobulins in IBS-D and SIBO
Non-published manuscript, authors:
Leonard B. Weinstock, MD, FACG, Bruce P. Burnett, PhD, Trisha L. Myers, PA-C
Study purpose
- Review the mechanism of action and efficacy of serum bovine immunoglobulins (SBI) in the treatment of IBS-D and SIBO.
Intervention:
- Review, non-systematic.
Main Results:
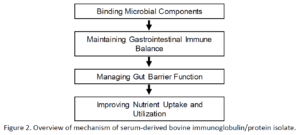
- What is the function of serum bovine immunoglobulins?
- “Immunoglobulins which bind to microbial components (i.e. endotoxins) to restore a normal immune balance and reduce inflammation.”
- Use of these immunoglobulins may interrupt a self-perpetuating cycling seen in dysbiosis and IBS.
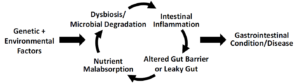
- Figure 1. The cycle of enteropathy caused by genetic and environmental factors which leads to dysbiosis, intestinal inflammation, leaky gut, and nutrient malabsorption.
- Toll-like receptors (TLRs) are largely responsible for binding microbial degradation products to assure an appropriate host immune response to antigens [18]. During dysbiosis, however, continual TLRs activation and expansion of T cell populations in the gut-associated lymphoid tissue (GALT) in response to repeated antigen penetration and presentation could lead to a cycle of inflammation and altered gut barrier function [19]. The resultant inappropriate and continual inflammation can lead to down-regulation of tight- and adherens-junction protein expression which can alter the proper uptake of salts and water [20,21]. In addition, chronic intestinal inflammation can lead to greater apoptosis of enterocytes [22] associated with decreased nutrient uptake and utilization [23]. With continued dysbiosis and microbial degradation, this can become a perpetual cycle of enteropathy that leads to so-called functional bowel disorders and in genetically susceptible individuals, diseases such as Crohn’s disease.
- In short, these immunoglobulins can help prevent immune activation
- Therefore, the presence of these microbial antigens creates a situation where SBI therapy can bind and prevent their penetration into the lamina propria which initiates immune activation.
- A more simplified mechanism….
- The authors cite numerous supports showing increase inflammation in those with IBS as compared to healthy controls
- In a population that was age and sex-matched, for example, mean serum levels of IL-6 and TNF-α in IBS-D patients (N = 63) were significantly higher than in healthy controls (N= 62), but there was no significant difference in the serum levels of anti-inflammatory IL-10 [56].
- Similar findings were found in a population of Chinese patients (42 IBS-D patients; 20 healthy controls) where TNF-α and IL-8 protein levels were significantly higher in the peripheral blood monocytes of IBS-D patients compared to healthy controls, whereas IL-10 levels were significantly reduced [57].
- In another study of sera and stool samples from post-infectious-IBS (n = 16), idiopathic-IBS (n = 44) versus healthy (n = 40), there were significantly higher levels of chemotactic chemokines monocytes chemotactic protein-1, macrophage inflammatory protein-1β, and chemokine (C-X-C motif) ligand 16 [58].
- In addition, pro-inflammatory cytokines (IFN-γ, IL-1β, and TNF-α) were significantly higher in IBS patients. Similar to other study findings, IL-10 was significantly higher in the healthy compared to IBS patients.
- O’Sullivan et al [60] found that mast cells (MCs) were significantly increased in the cecum of IBS patients compared with controls.
- This inflammation also correlated with depression and anxiety; gut-brain.
- Serum and tissue levels of inflammatory cytokines also seem to track with anxiety and depression in functional bowel conditions. Symptom scores of IBS-D patients were also significantly correlated with TNF-α and IL-8 in one study: TNF-α with depression; IL-8 with anxiety [62].
- Gao [63] found similar results in a population of 12 patients with normal compared to 16 with elevated anxiety-depression scores. Blood and sigmoid colon mucosa levels of IL-1β and the proportion of IL-1β-positive cells were significantly higher IBS-D patients experiencing anxiety and depression.
- These immunoglobulins are “~92% protein purified from USDA-approved food-grade plasma with >50% IgG. The specially formulated protein isolate also includes ~1% IgA, 5% IgM, and other proteins typically found in plasma and milk.”These immunoglobulins are free of casein, whey, lactose, soy, gluten, and dye.”
- Stomach acid does not degrade these immunoglobulins
- Oral immunoglobulins and non-absorbed and can survive the gastric environment and the entire length of the intestinal tract.
- Serum-bovine immunoglobulins have been shown to bind to a number of antigens associated with dysbiosis and poor barrier function
- has been tested for its binding to numerous microbial antigens including LPS (i.e. bacterial endotoxin), Clostridia difficile toxin A and B, peptidoglycan (bacterial antigen), flagellin (antigen from gram-negative bacteria), zymosan (yeast antigen), cyclic diadenylate monophosphate (bacterial antigen), cytosine-phosphate-guanine (bacterial DNA sequence), Pam3CSK4 (LPS mimic), and N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP, bacterial antigen) [111].
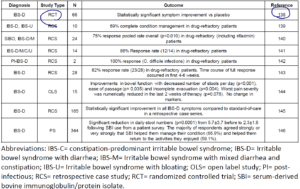
- What does the clinical data say? See table below. Here is my summary
- One randomized clinical trial
- – result = helpful [138]
- One retrospective trial in patients who were non-responsive to SIBO treatment (and/or other IBS treatments)
- – result = helpful see prior summary here and abstract here [140]
- 4 retrospective case-series (not the same quality as RCT) in otherwise non-responsive IBS-D
- In a large survey of roughly 600 IBS patients
- – 67% found SBI to be significantly helpful.
- Table: Clinical Study Data for SBI in Functional Bowel Disorders
- One randomized clinical trial
- Dosing and duration guidelines
- Dose – 5 grams/day for mild cases, moderate/severe cases should use 10 grams per day
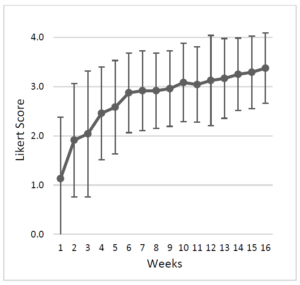
- Duration – the response should be noted by 1-2 weeks. Plateau may be achieved by 6 weeks.
- Based upon study [143].
- Note this study found an 82% response rate in drug-refractory IBS within 6 weeks (note this was non-controlled so this is likely misleadingly high).
Additional Results:
- Some evidence suggests that the presentation of food antigens can trigger leaky gut
- Fritscher-Ravens et al [79] demonstrated using confocal microscopy in IBS patients that when they were exposed to antigens such as milk, wheat, yeast, and soy mixed with fluorescein, within 5 minutes the fluorescein could be visualized draining between increased gaps and spaces in the epithelial layer in the duodenum.
- It should be obvious from following our newsletter that the gut has a far-ranging impact. But its work including these references none the less.
- IBS is comorbid with SIBO, fecal incontinence, gastrointestinal reflux, functional dyspepsia, fibromyalgia, chronic pelvic pain, anxiety, depression, chronic fatigue syndrome and headaches [83, 84, 85]. SIBO is comorbid with liver disease [80, 81], obesity [86], restless legs syndrome [87], rosacea [88]. There is also an association of SIBO with rheumatoid arthritis [89], HIV-induced immunosuppression [90], chronic pancreatitis [91], cystic fibrosis [92], diabetes [93] and cardiovascular autonomic neuropathy [94]. SIBO is also comorbid with several GI conditions and diseases such as Crohn’s disease, celiac disease, IBS and short bowel syndrome [95].
- It appears that these supplemental immunoglobulins are not absorbed but may help improve nutrient absorption.
- In healthy human subjects (N = 42), Shaw et al [132] examined the plasma amino acid profile following SBI administration and whether bovine IgG was present in stool or blood with increasing, multiple doses of the protein isolate. Plasma concentrations of essential amino acids and tryptophan were significantly increased over placebo after ingestion of 5 g, 10 g, or 20 g of SBI.
- Total amino acids were significantly increased at 10 g and 20 g doses. There was a significant difference in plasma concentrations between all SBI doses and placebo for essential amino acids and tryptophan. Bovine IgG was found in stool samples following multiple doses of SBI consistent with earlier data on other IgG preparations [109], but quantifiable levels were not found in plasma 90 minutes after administration of single or multiple doses.
Limitations:
- More clinical trial data here would be nice. That said, I feel there to be enough evidence to justify a trial on these immunoglobulins.
Authors Conclusion:
- “A non-absorbed, gut-directed therapy that improves leaky gut, reduces uncontrolled immune dysfunction and reestablishes a balanced immunity is desirable. It is with this goal that SBI may be desirable.”
Interesting Notes:
- Remember, there is more to the underlying cause of SIBO than just motility. It is my belief that local immune function is not given enough attention.
- Innate mechanisms that prevent bacterial overgrowth include … secretory IgA (sIgA) production in the intestine which binds to bacteria and other antigens…
Clinical Takeaways:
- Serum bovine immunoglobulins provide a novel mechanism for healing the gut, by binding toxins and reducing immune activation
- Preliminary data shows them to be effective even in otherwise non-responsive patients
- A dose of 10, perhaps only 5 grams per day is effective
- 2 weeks is adequate time to have an initial response
Dr. Ruscio Comments
This is an exciting new compound worth experimenting with in otherwise non-responsive patients.
The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study.
https://www.ncbi.nlm.nih.gov/pubmed/30060266
Study purpose
- To assess if a gluten-free diet would effect thyroid antibodies or hormone production in non-hypothyroid women with positive thyroid antibodies.
Intervention:
- 34 women with positive thyroid antibodies were divided into 2 groups
- Group A: gluten-free diet for 6 months
- Group B: no dietary change
Main Results:
- The gluten-free diet
- Reduced thyroid antibodies, by roughly 200 points
- Increased vitamin D levels, by roughly 5 points
- The gluten-free diet reduced thyroid antibody titers, as well as slightly increased 25-hydroxyvitamin D levels and the SPINA-GT index.
- In group A, the impact on TPOAb and TgAb titers correlated with the changes in the SPINA-GT index, whereas the impact on TPOAb with the changes in 25-hydroxyvitamin D levels.
- Did not have any effect on thyroid hormone levels
- The gluten-free diet did not affect thyrotropin, free thyroid hormones, Jostle’s thyrotropin index, and the SPINA-GD index
- Improvements in TPO antibodies correlated with Tg antibodies, my thought: suggesting perhaps tracking just TPO could suffice.
- the impact of treatment on TPOAb titers correlated with the changes in TgAb titers
- Specific changes in antibody levels
- GF group saw a 200-point decrease in either antibody
- the group not changing its diet experienced a 40-point increase. See below
- Changes in vitamin D
- 5 points, I question if this is enough to make a substantial impact on antibody levels
Additional Results:
- Part of the mechanism through which a gluten-free diet improves thyroid autoimmunity could be via increasing vitamin D levels. The authors also speculate that a GF diet could have improved selenium absorption.
- Those with the highest antibodies saw the greatest improvements in antibodies. This hints at an important concept of a low of diminishing returns regarding a gluten-free diet’s impact on thyroid autoimmunity. This is very important for clinicians to understand, don’t beat your patients to death with a gluten-free diet.
- Secondly, the effect on antibody titers was strongest in patients with the highest antibody titers
- A gluten-free diet may not affect peripheral metabolism
- the gluten-free diet does not affect the peripheral metabolism of thyroid hormones
Limitations:
- *IMPORTANT* The group selected would likely be most prone to be reactive to gluten. Why? It included those with transglutaminase antibody positivity.
- To be admitted to the study, they were required to have
- (a) positive TPOAb (>100 U/mL),
- (b) the reduced echogenicity of the thyroid parenchyma on thyroid ultrasonography;
- (c) normal thyroid function (thyrotropin levels in the range between 0.4 and 4.5 mU/L, free thyroxine in the range between 10.0 and 21.0 pmol/L and free triiodothyronine in the range between 2.6 and 6.5 pmol/L) and
- (d) incidentally found positive anti-tissue transglutaminase antibodies without clinical symptoms of coeliac disease.
- To be admitted to the study, they were required to have
- It was also found that after the intervention period, more than ½ of those in the GF diet group experienced a normalization of their anti-Tg antibodies, whereas there was no improvement in the group not changing their diet. Suggesting those with anti-Tg antibodies may be more prone to improve from a gluten-free diet.
- At the end of the study, positive anti-tissue transglutaminase antibodies were present in sera of 6 patients (38 %) from group A and in all 18 patients from group B.
Authors Conclusion:
- “The obtained results suggest that the gluten-free diet may bring clinical benefits to women with autoimmune thyroid disease.”
Interesting Notes:
- Those with thyroid autoimmunity have increased risk of celiac, true.
- A pooled analysis, including 6024 patients with autoimmune thyroiditis, found a markedly increased prevalence of biopsy-confirmed coeliac disease, allowing the authors to conclude that all patients with autoimmune thyroiditis should be screened for the presence of coeliac disease
- However, when we look at this pooled analysis (meta-analysis) mentioned above, we see the prevalence is 1 in 62. https://www.ncbi.nlm.nih.gov/pubmed/27256300 This is increased, but it’s important not to erroneously believe this means Hashimoto’s = celiac.
- What about NCGS? Well, as we have covered in the past, about 9% of those with NCGS were found to have Hashimoto’s in the largest trial I know of examining this connection
- So, yes, there is an association and it is significant. This applies to both celiac and NCGS, but it is not the majority so we should not hold all Hashimoto’s patients to a celiac standard unless we have built a case for this.
Clinical Takeaways:
- A gluten-free diet may lead to roughly a 200-point improvement in thyroid antibodies
- This effect may not apply as fully to those without transglutaminase antibodies
- The higher one’s thyroid antibodies the larger the potential for improvement from a GF diet
- Improvement in vitamin D and selenium absorption are potential mechanisms
Dr. Ruscio Comments
Not much to add here, other than a caution to not overly recommend gluten avoidance. Why? It is my belief that improving one’s gut health is crucial for thyroid function. In optimizing gut health, gluten-free is very helpful for some, but not for others. It’s more important to find the diet that will lead to the greatest symptomatic improvement in your patients. For some, other dietary factors will be more important to mind and this should be offset by a relaxation of the recommendation of gluten avoidance so as not to unnecessarily burden someone with frivolous dietary restrictions.
Fluctuation of zonulin levels in blood vs stability of antibodies.
https://www.ncbi.nlm.nih.gov/pubmed/28883692
Study purpose
- Evaluate the accuracy of zonulin testing.
Intervention:
- Serum zonulin levels were compared to zonulin antibody levels, in celiac patients versus controls. Other measures of leaky gut were also measured.
- This study was conducted to assess the variability or stability of zonulin levels vs IgA and IgG antibodies against zonulin in blood samples from 18 controls at 0, 6, 24 and 30 h after blood draw. We also measured zonulin level as well as zonulin, occludin, vinculin, aquaporin 4 and glial fibrillary acidic protein antibodies in the sera of 30 patients with celiac disease and 30 controls using enzyme-linked immunosorbent assay methodology.
Main Results:
- Zonulin antibodies appeared more stable than serum zonulin
- The serum zonulin level in 6 out of 18 subjects was low or < 2.8 ng/mL and was very close to the detection limit of the assay. The other 12 subjects had zonulin levels of > 2.8 ng/mL and showed significant fluctuation from sample to sample. Comparatively, zonulin antibody measured in all samples was highly stable and reproducible from sample to sample.
- Elevations of zonulin antibodies may more closely correlate with celiac than does serum zonulin.
- Celiac disease patients showed zonulin levels with a mean of 8.5 ng/mL compared to 3.7 ng/mL in controls (P < 0.0001).
- Elevation of zonulin level at 2SD above the mean was demonstrated in 37% of celiac disease patients, while antibodies against zonulin, occludin and other tight junction proteins were detected in up to 86% of patients with celiac disease.
- Essentially, serum zonulin has too great a fluctuation whereas zonulin antibodies are more stable.
- We studied possible variability in zonulin levels vs measuring antibodies against zonulin and other tight junction proteins in the blood. We found that fluctuations in zonulin level from hour-to-hour and day-to-day were too great to recommend it for assessing intestinal permeability. Measurement of IgG and IgA antibodies against tight junction proteins in controls and in celiac disease patients proved to be very stable and reproducible, and we recommend this method for such an assessment in future studies.
- Zonulin was positive in 33% of celiac patients whereas zonulin antibodies were positive in 67% of celiac patients.
- At 2 standard deviations above the mean of control or a serum zonulin level of 7.1 ng/mL, 10 out of the 30, or 33% of the CD patients exhibited elevations in zonulin levels (P < 0.0001) as well as in zonulin IgA and IgG antibody levels. However, we found that an additional 10 CD patients or a total of 20 (67%) produced antibodies to zonulin without having a significant elevation in serum zonulin levels.
Additional Results:
- Zonulin correlated with vinculin IgA and IgG antibodies
- Zonulin levels also had statistically significant correlations with vinculin IgA and IgG antibodies, and the magnitude of the relationship was likewise better with vinculin IgA antibodies
- A number of studies have shown zonulin in the blood to correlate with various conditions. Note: I am still a bit tenuous about serial testing of ‘leaky gut’ as I am unclear how this changes treatment in any meaningful way.
- During the past 3 years, a few published studies have shown elevations of zonulin levels in the blood of subgroups of patients with type 1 diabetes [41], metabolic syndrome [42], polycystic ovary syndrome [43], and type 2 diabetes [44]. Based on these publications, several clinical laboratories are now offering measurement of zonulin levels in the blood as a biomarker of gut barrier assessment and autoimmunities.
- Zonulin levels appear to be highly in flux
- Functional medicine may be rushing to a marker before it’s fully validated or ready for the clinical setting. I credit the authors for issuing this caution.
- offering serum zonulin levels as a diagnostic indicator mainly stems from a paper published by Sapone et al [41] in 2006 which demonstrated a correlation with zonulin upregulation and increased gut permeability in a subgroup of patients with type 1 diabetes.
- data on which the conclusions were drawn merit further analysis before they become the sole basis for future diagnostic utility.
- In fact, the authors of this paper cite the original study suggesting zonulin as a marker of leaky gut. The authors of the original study remarked the zonulin test isn’t ready for routine clinical practice yet. Here is another area where you can simplify your practice model and save patients money.
- While these numbers are very useful in the setting of research analysis, they are insufficiently correlated to be applied to diagnostic medical use.
Limitations:
- To my knowledge, this is the first study of its kind and I’d like to see the results replicated by others, especially those who don’t own a lab specializing in this testing.
Authors Conclusion:
- Due to its fluctuation, a single measurement of zonulin level is not recommended for assessment of intestinal barrier integrity. Measurement of IgG and IgA antibodies against zonulin, occludin, and other tight junction proteins is proposed for the evaluation of the loss of intestinal barrier integrity.
Clinical Takeaways:
- Zonulin testing is interesting but may not be ready for clinical practice yet
- Zonulin antibody testing might be more accurate than serum zonulin testing
- Further research appears required before adaptation in clinical practice
Dr. Ruscio Comments
My clinical experience, although limited, in looking at zonulin levels (in the stool) is they are hit or miss regarding if they correlate with a patients presentation. This study and the original zonulin study the authors’ reference corroborate my experience. While I have not performed an exhaustive review of the data here, it does appear that there is not yet a solid justification leg for zonulin testing to stand on in clinical practice. In my experience, a key to success in functional medicine is knowing what bells and whistles not to get pulled into, this appears one such bell. The correlation between pathology and zonulin may be less than 50%, as just a rough estimate from some of the papers. This does not inspire huge amounts of faith in guiding clinical decision making.
Rapid-Fire Research – Ultra-Concise Summaries of Noteworthy Studies
A Low FODMAP Gluten-Free Diet Improves Functional Gastrointestinal Disorders and Overall Mental Health of Celiac Disease Patients: A Randomized Controlled Trial.
https://www.ncbi.nlm.nih.gov/pubmed/30081576
- 50 celiac patients who still had symptoms after going gluten-free were then put on either a low FODMAP diet or a control diet (no change).
- Only the low FODMAP diet group, experiences;
- Improved digestive symptoms
- Large improvements in general wellbeing
Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome.
https://www.ncbi.nlm.nih.gov/pubmed/24833942
- Roughly 60 patients with IBS-D were given either 5g/day or 10g/day of immunoglobulins or placebo for 6 weeks.
- The placebo was soy protein, this should be noted as it may skew results to make the treatment appear relatively more effective.
- Results:
- The immunoglobulin groups reported significant improvement symptom frequency, over the placebo group.
- A non-significant trend emerged with improved symptoms severity in the 10g/day group.
- While individual symptoms and severity did improve, total IBS symptoms scores were similar between groups.
- Significant improvement in hemoglobin concentrations (note: suggesting improved iron absorption).
- 4 cases reported adverse events, dose did not matter.
Association of Thyroid Hormone Therapy With Quality of Life and Thyroid-Related Symptoms in Patients With Subclinical Hypothyroidism: A Systematic Review and Meta-analysis.
https://www.ncbi.nlm.nih.gov/pubmed/30285179
- 21 of 3,088 initially identified publications met the inclusion criteria, with 2,192 adults randomized.
- Treatment with thyroid hormone;
- brought thyroid hormone level into a normal range
- had no effect on quality of life symptoms
- had no effect on thyroid-related symptoms
- After treatment (range, 3-18 months), thyroid hormone therapy was associated with lowering the mean thyrotropin value into the normal reference range compared with placebo (range, 0.5-3.7 mIU/L vs 4.6 to 14.7 mIU/L) but was not associated with benefit regarding general quality of life (n = 796; SMD, -0.11; 95% CI, -0.25 to 0.03; I2=66.7%) or thyroid-related symptoms.
- Risk of bias was low and evidence quality was moderate to high.
- Conclusion ‘in non-pregnant individuals, thyroid hormone should not be used for those with sub-clinical hypothyroidism’.
- use of thyroid hormone therapy was not associated with improvements in general quality of life or thyroid-related symptoms. These findings do not support the routine use of thyroid hormone therapy in adults with subclinical hypothyroidism.
Thyroxine and triiodothyronine content in commercially available thyroid health supplements.
http://www.ncbi.nlm.nih.gov/pubmed/23758055
- Many natural ‘thyroid support’ supplements may contain actual thyroid hormone.
- The majority of dietary thyroid supplements studied contained clinically relevant amounts of T4 and T3, some of which exceeded common treatment doses for hypothyroidism. These amounts of thyroid hormone, found in easily accessible dietary supplements, potentially expose patients to the risk of alterations in thyroid levels even to the point of developing iatrogenic thyrotoxicosis. The current study results emphasize the importance of patient and provider education regarding the use of dietary supplements and highlight the need for greater regulation of these products, which hold potential danger to public health.
- Why does this matter?
- Someone who needs medication may be relying on a supplement to obtain the hormone they need. This inherently is fine, however, it is likely much cheaper to use a hormone especially if one has insurance coverage. Also, caution should be exercised to prevent overdosing.
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next practitioner question of the month.

Like what your reading?
Please share this with a colleague and help us improve functional medicine

Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!