Treating Clinician: Robert Abbott, MD
Patient Info:
- Nathan, 31 y/o, male
- Previous Dx
- n/a
- Rx
- n/a
- Chief Complaints
- Constipation, 4-Intermittent
- Lack of weight loss, 5-Intermittent
- Pruritus ani, 4-Intermittent
- Fatigue, 4-Intermittent
- Bloating, 3-Occasional
- Other symptoms
- Allostatic load, moderate – fatigue, insomnia
Initial impression:
- Nathan is a 31 y/o male, on Keto diet, with a good lifestyle, demeanor, and outlook.
- Dx/Rx:
- n/a
- Previous Testing:
- H. pylori IgA abs.
- Onset:
- All symptoms started after 9 months of antibiotic treatment in his 20s.
- Family History:
- n/a
- Prior Treatments:
- Probiotics, HCl – helpful
- Notes/DDX:
- Likely has mild degree of GI dysbiosis and might have H. pylori or APCA, causing need for HCl. May also be overwashing.
- DDx:
- SIBO
- Dysbiosis, GI infection
- Histamine intolerant
- Gastritis
- Dermatological candida
- Hg tissue burden
- Prognosis:
- Good to excellent
- Previous Diets:
- Keto – helpful
- Fasting – helpful
- Veggies – help constipation
Testing:
- Tests ordered
- GI-Map stool test
- APCA Antibodies
- CBC w/ differential
- Rationale
- Stool testing to affirm if HP is still present, this could/should also be paired with HP breath test.
- APCA antibodies as a potential window into gastric inflammation and HCl production capacity.
Recommendations:
- Diet:
- Continue your previous Ketogenic diet. Do your best to be compliant, but you do not need to be perfect.
- Continue intermittent fasting. 2 days per week, fasting 14-18 hours is a general aim to steer towards.
- Lifestyle:
- Reduce/eliminate use of soap on the body. You can continue to use on hands and hair. Give this 4 weeks and then reevaluate your skin/itchiness.
- Reduce coffee consumption, as you have already started doing
- Please spend some time in the sun at least a few days per week with as much skin exposed as possible.
- NRT: vit D/K, Multi, Fish Oil
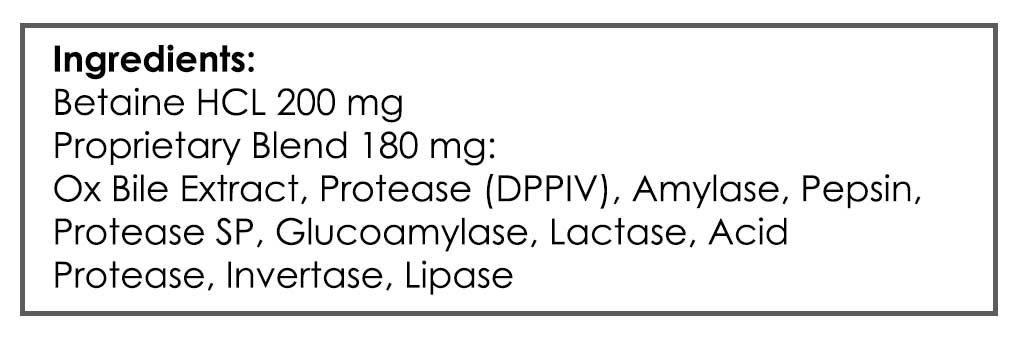
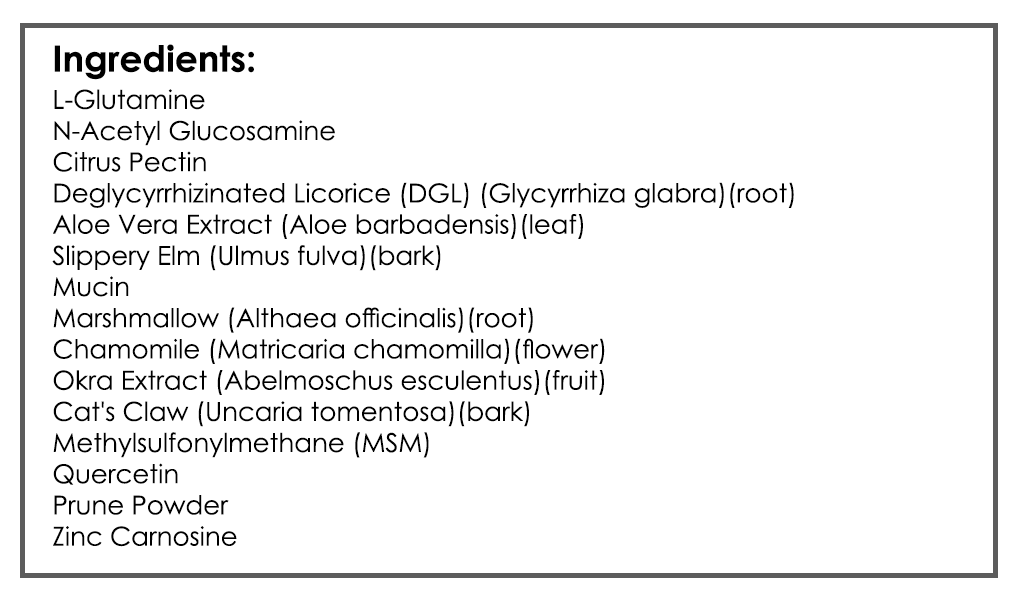
- GI: Digestive Acid HCl, Gut Healing Nutrients (note: experiment with higher end of dose range with HCl)
- Rationale
- Coffee can flare gastritis, which I suspect. GI reparative nutrients may also help with gastric healing.
- Follow up: 4-5 weeks
Subjective Assessment:
- Improved:
- Digestion, 2, Improvements overall by introducing ginger and enzymes
- Vitamin D, Getting 20 minutes of sun 3 to 4 times a week
- Inability to lose weight – dropped from 268 lbs to 232 lbs
- Same:
- Low energy/fatigue, 3
- Muscle stiffness and exercise recovery, 3
- Worse:
- Constipation, 2, Gets better with bone broth and the gut repair
Lab Interpretation:
- Stool Test – GI Map (January)
- H. pylori
- H. pylori
- Stool test – GI Map (August)
- H. pylori CLEARED
- H. pylori CLEARED
- Note: Patient did not complete the other tests
Impression:
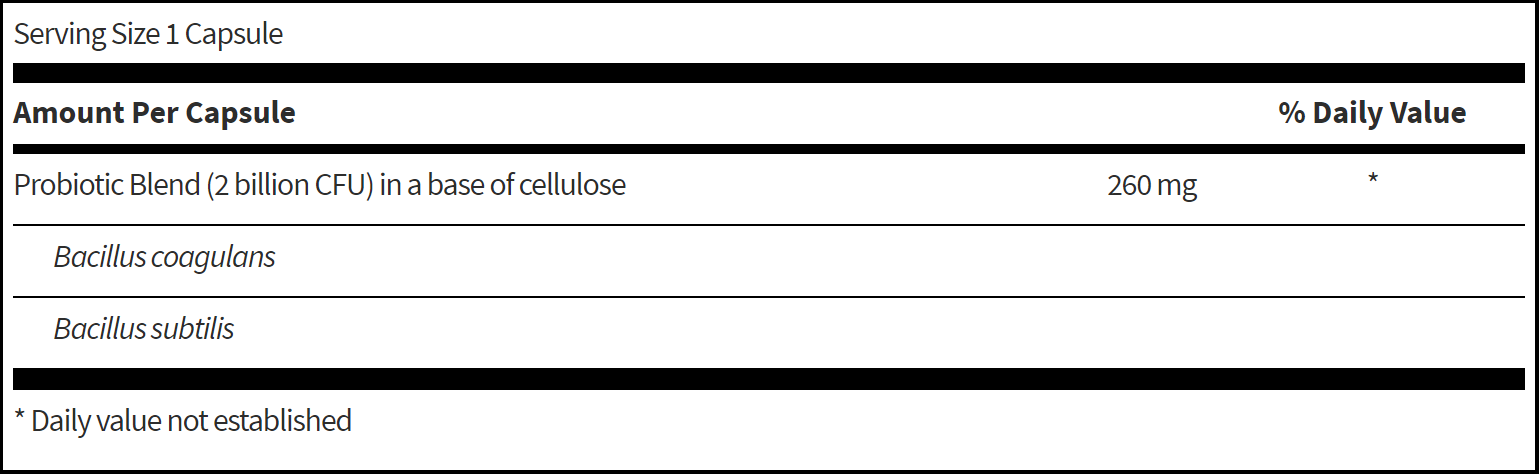
- Today is doing great overall, much improved. Was not on probiotics at first test, was on second test, HP was cleared during this time. Today will start on 3 probiotics together; lacto-biff, boulardii, and soil-based.
Recommendations:
Starting
- GI: Lacto-Biff, S. boulardii, Soil-based probiotics
- Consider running the APCA test for stomach autoimmunity soon
Follow up: 2-3 months
Dr. Ruscio’s Comments
This is a GREAT case showcasing how H. pylori, ostensibly when combined with diet and lifestyle changes, can be addressed with probiotics as a stand-alone therapy. We are still working to address lingering symptoms but I wanted to share a clear example of how we might be overusing herbal or pharmaceutical antibiotics.
Research supports this finding as well
In a systematic review, Saccharomyces boulardii alone was shown to eliminate H. pylori infection in 12% of cases, though more research is needed to confirm this result. See more info here.
Executive Summary:
- Reduced production of pancreatic enzymes
- Affecting roughly .005% of the population
- Causing gas, bloating, pain, diarrhea, potential weight loss
- Treated with titrated pancreatic enzymes
- Predominantly caused by lifestyle (alcohol, smoking…) and idiopathically.
- Can, rarely, be caused by pancreatic cancer or cystic fibrosis.
- Referral to GP or GI should be performed to be on the safe side.
What is EPI (Exocrine Pancreatic Insufficiency)?
- Insufficient release of pancreatic enzymes which leads to digestive symptoms and malabsorption.
Symptoms
- gas and bloating
- abdominal pain
- greasy, oily bowel movements (stools)
- diarrhea (very loose stools)
- foul-smelling stools
- unexplained weight loss
Prevalence
- 8 per 100,000 for males and 2 per 100,000 for women (roughly 0.005% of population) [1]
Etiology & Pathophysiology
Causes of EPI
- Chronic pancreatitis
- Most common cause of EPI
- Causes of pancreatitis: alcohol, smoking, autoimmune genetics
- *a significant number (20%) remain idiopathic after investigation*
- Autoimmune marker: Serum immunoglobulin G4 (IgG4)
- Pancreatic cancer
- Cystic fibrosis
- Removal of some or all of the pancreas
- Surgery: Gastric, pancreatic, or small bowel resection to other parts of the digestive tract (such as the intestines or stomach)
Associated Risks
- Deficiencies of fat-soluble vitamins and other micronutrients, and concomitant bone disease.
- Pancreatic cancer: prevalence .02%
- Cystic fibrosis: prevalence .009%
Referrals
- DrR’s thoughts:
- Given the population this test is often being performed in, IBS, and especially if IBS-D, the incidence of false positives may be higher. Also, when considering a significant number of cases, perhaps the majority, are due to lifestyle or idiopathic, it makes the likelihood of cancer or cystic fibrosis quite low. However, to be prudent a referral to GP or GI should be made for follow-up investigation.
- Only Canadian guidelines give referral recommendations for Pancreatic Exocrine Insufficiency (PEI)
- When and how should the primary care physician refer the patient to a gastroenterologist?
- Referral should be considered when the patient shows evidence of abnormal tests (e.g., FE-1, plasma fat-soluble vitamin deficiencies) following screening, and/or when the patient experiences ongoing symptoms, such as steatorrhea or weight loss.
- It is particularly important for patients to be referred to a specialist for further diagnostic workup if PEI is diagnosed in the absence of any previously established predisposing conditions, as PEI can often be the first clinical manifestation of pancreatic cancer or Chronic Pancreatitis (CP).
- For this reason, obtaining a CT scan to exclude pancreatic cancer or CP after diagnosing PEI while awaiting specialist consultation should be strongly considered.
- For patients with symptoms suggestive of PEI, such as steatorrhea or unexplained weight loss, the PCP should document duration and nature of symptoms, along with any abnormal nutritional parameters and markers of malnutrition. If a trial of PERT has been attempted, dosing, duration of treatment, and clinical outcomes should be reported in the referral letter.
- When and how should the primary care physician refer the patient to a gastroenterologist?
Testing
- Best marker weighing cost and invasiveness = fecal elastase
- Response to treatment may be adequate to diagnose
- ‘clinical suspicion is often sufficient to make the diagnosis without formal fecal fat measurement in the proper clinical context, with titration of pancreatic enzymes to improve symptoms’
False positives
- Diarrhea may cause false positive
- ‘Acute or chronic diarrhea may give falsely high PE-1 results and may be a low-risk diagnosis due to benign diarrhea.’
Conventional Labs
Functional Labs
Treatment
- Titrate to find optimal dose for symptomatic response.
OTC
- Pancreatic Enzymes (standard OTC): 2-8 capsules per meal. Ideally, take part of your dose at the start of the meal and the rest mid-meal. Do your best and don’t worry if not perfect.
RX
- Creon Rx: 1-3 capsules per meal. With meals, any time.
Guidelines
- Lipase is often the enzyme used to determine dosage, but all pancreatic enzymes should be supplemented together (protease, amylase, lipase)
- 30,000-90,000 of lipase per meal is the target dose, adjusted based upon patient response
- Take with first bites of meal
- I don’t feel it necessary to give weight-based dosages, because the dose needed for an individual can vary so it seems prudent to standardize to a dose titration
Closing Thoughts
- This dx could easily scare patients if not framed correctly, be cognizant.
- Testing does NOT appear necessary, testing is a consideration but not a necessity.
- False positives occur, don’t over interpret, use other supporting data to affirm veracity.
- Referral may be indicated. Consider this pragmatic approach; if elastase remains elevated upon repeat testing once diarrhea is resolved, you have qualified the referral.
Does a glucose-based hydrogen and methane breath test detect bacterial overgrowth in the jejunum?
Neurogastroenterol Motil. 2018 Nov;30(11):e13350. doi: 10.1111/nmo.13350. Epub 2018 Apr 23.
- SIBO breath testing may more accurately be described as testing for small intestinal dysbiosis, rather than for overgrowth.
- This may not make a discernible impact on treatment as it appears challenging to effect one without affecting the other.
Effect of Vitamin D Supplementation on Fecal Microbiota: A Randomized Clinical Trial
Nutrients 2019, 11(12), 2888; https://doi.org/10.3390/nu11122888
- Vitamin D impacts the microbiota
- Our findings suggest that vitamin D supplementation has some distinct effects on fecal microbiota. Future studies need to explore whether these effects would translate into improved clinical outcomes.
- Here is a cherry-pick, ‘vitamin D has a large impact on microbiota!’
- Here is the full story, not so clear-cut, the methodology makes a sizable impact on the finding….
- Even more interesting is that other measure found reduced richness
- The alpha diversity of the microbiota profile at baseline was not different between the vitamin D and placebo groups (p = 0.9). Similarly, at follow-up, there were no significant differences in microbiota richness and evenness between the vitamin D and placebo groups. However, the vitamin D-supplemented group showed a reduction in bacterial richness at follow-up compared to the baseline (p = 0.050), whereas no significant differences were observed in the placebo group.
- The alpha diversity of the microbiota profile at baseline was not different between the vitamin D and placebo groups (p = 0.9). Similarly, at follow-up, there were no significant differences in microbiota richness and evenness between the vitamin D and placebo groups. However, the vitamin D-supplemented group showed a reduction in bacterial richness at follow-up compared to the baseline (p = 0.050), whereas no significant differences were observed in the placebo group.
- There is still much we have to learn with measuring the microbiota. Unless you go to a direct to consumer microbiota testing company (they have it all figured out…) – hope you are registering the sarcasm :).
The effects of high doses of vitamin D on the composition of the gut microbiome of adolescent girls
https://clinicalnutritionespen.com/article/S2405-4577(19)30494-2/fulltext#.XdCa5J39XZs.twitter
- This study provides more of the same; impact but the meaning of which is unclear.
- The expression fold changes for Enterococcus, Bifidobacterium, Lactobacillus, Bacteroidetes and Firmicutes were; 1.05, 1.20, 0.76, 0.28 and 1.50 respectively. Bacteroidetes and Lactobacillus fell by 72% (P < 0.0001) and 24% (P = 0.006) respectively, whilst Firmicutes and Bifidobacterium were increased by 1.5 (P < 0.0001), 1.2 (P < 0.0001) fold after supplementation.
- Conclusion: Our results suggested that a high dose supplementation of vitamin D alter the human gut microbiome composition. Future studies are required for a better understanding of the mechanisms by which vitamin D affects the gut microbiome.
What is the most tenable conclusion regarding vitamin D and the microbiota?
Hmm, if there was only a technique we could use to parse data according to which has the most meaning. Would this be the scientific method and the guidelines of the evidence based model? Could that mean that if in clinical trials IBS patients see reduced symptoms upon vitamin D supplementation, then we could somewhat safely conclude that vitamin D has a beneficial effect on the microbiota but we are not yet able to fully measure or understand it yet? Seems probable to me.