Practitioner Research Review – February 2019
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
In Today’s Issue
Research
- Vitamin D Treatment in Patients with Hashimoto’s Thyroiditis may Decrease the Development of Hypothyroidism.
- Effects of Long-Term Combination LT4 and LT3 Therapy for Improving Hypothyroidism and Overall Quality of Life.
- Non-celiac gluten sensitivity: All wheat attack is not celiac.
- Rapid-Fire Research – Ultra concise summaries of noteworthy studies
- The role of low fermentable oligosaccharides, disaccharides, monosaccharides, and polyol diet in nonceliac gluten sensitivity
- A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects
- Recommendations for treatment of hypothyroidism with levothyroxine and levotriiodothyronine: a 2016 position statement of the Italian Society of Endocrinology and the Italian Thyroid Association.
- Effectiveness and safety of calcium and vitamin D treatment for postmenopausal osteoporosis.
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however, the research studies are a collection of the most clinically meaningful research that has been published recently.
Vitamin D Treatment in Patients with Hashimoto’s Thyroiditis may Decrease the Development of Hypothyroidism.
https://www.ncbi.nlm.nih.gov/pubmed/28697689
Study Purpose
- Essentially, assess the impact of vitamin D on thyroid antibodies
Intervention:
- 75 patients with Hashimoto’s thyroiditis and 43 healthy individuals.
- Vitamin D deficiency is defined as a 25-hydroxyvitamin D (25(OH)D3) concentration less than 20ng/mL.
- Vitamin D deficient patients were given 50,000 units of 25(OH)D3 weekly for eight weeks.
Main Results:
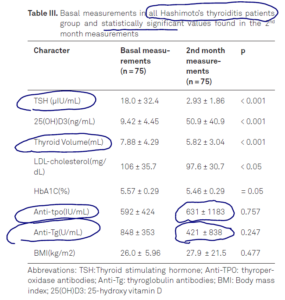
- Patients with Hashimoto’s thyroiditis had significantly lower vitamin D concentrations compared with the controls (9.37±0.69 ng/mL vs 11.95±1.01 ng/mL, p < 0.05, respectively).
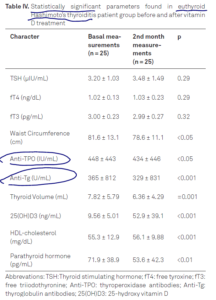
- Thyroid autoantibodies were significantly decreased by vitamin D replacement treatment in patients with euthyroid Hashimoto’s thyroiditis.
- Note: the decrease in antibodies did not occur in all treated patients (table III), only in a subgroup of patients (Table IV).
- Also, the decrease in antibodies was not clinically meaningful in my opinion
- So, yes vitamin D can help, but it is not a cure-all for Hashimoto’s.
- Ironically, there was no correlation between vitamin D levels and thyroid antibodies.
- “We found no correlation between thyroid antibodies and 25(OH)D3”
- Thyroid volume decreased in the treatment group
- HDL cholesterol concentrations improved in the euthyroid Hashimoto group after treatment.
Limitations:
- Some patients were on thyroid medication, which could skew the association between vitamin D and antibodies.
Authors Conclusion:
- Vitamin D deficiency is frequent in Hashimoto’s thyroiditis and treatment of patients with this condition with Vitamin D may slow down the course of development of hypothyroidism and also decrease cardiovascular risks in these patients. Vitamin D measurement and replacement may be critical in these patients.
Clinical Takeaways:
- Vitamin D may improve thyroid autoimmunity. However, it may not help in all patients and even in those, it does help the effect may be non-meaningful clinically.
Dr. Ruscio Comments
Not much to add here other than the observation that understanding these nuances helps prevent one from making overreaching claims and conclusions. Or, said more simply, when you understand what treatment can and can’t do, you are less likely to fall into hyperbolic claims. Also, remember these findings should be weighted against the entire body of data.
Effects of Long-Term Combination LT4 and LT3 Therapy for Improving Hypothyroidism and Overall Quality of Life.
https://www.ncbi.nlm.nih.gov/pubmed/29863229
Study Purpose
- Compared T4 alone versus T4+T3 medication on hypothyroidism and quality of life in patients not responding to T4 alone.
Intervention:
- An observational retrospective study, 6 years in duration
- Medications compared: synthetic T4+T3 or desiccated thyroid extract T4+T3 (natural therapy)
- In patients not previously responding to T4
- Patients were started on a combination therapy if they complained of hypothyroid signs and symptoms despite optimal trials of LT4 monotherapy for at least 1 year, to achieve optimal normal TSH levels, preferably <2.5 μIU/mL based on recent evidence (37) and if they continued to have low normal FT3 (2.5–3 μIU/mL).
- Synthetic T3 Dosages – LT4/LT3 average dose was 75 μg/5 μg
- The starting dose of LT3 was 5 μg in conjunction with an appropriate decrease of 12.5 μg in LT4 to achieve the standard physiological circulating FT4:FT3 ratio of nearly 14:1.
- LT3 was titrated with the maximum 12.5 μg dosage to achieve physiologic and therapeutic FT3 levels along with symptom relief.
- None of the patients were treated with LT3 alone.
- Desiccated dosage – average dose 30mg
- The starting dose for DTE, with a known FT4:FT3 ratio of 4:1 was 15 mg and was titrated to obtain physiologic and therapeutic TSH, FT4, and FT3 levels along with symptom relief.
- Individual endocrinologists chose the smaller dosage when switching from LT4 to DTE and to prevent adverse effects of hyperthyroidism.
Main Results:
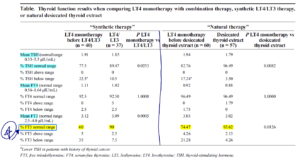
- Compared to when on, and not responding to, T4 alone:
- TSH levels were better in the majority (roughly 90%) of patients on combination therapy
- Compared with patients taking only LT4, 89.47% using synthetic therapy had therapeutic TSH (P < 0.05).
- Similarly, 96.49% using natural therapy had therapeutic TSH (P < 0.05).
- Symptoms were better in the majority (roughly 90%) of patients on combination therapy
- >92% answered feeling “excellent, very good, or good” when questioned about their health while undergoing thyroid replacement compared with levothyroxine alone.
- Adverse events appeared low, 6 of 100. There were no cardiovascular side effects
- Six patients were excluded because they had ceased therapy.
- In our study, at least 6.7% of the 100 patients complained of palpitations and anxiety and had confirmed TSH <0.35 μIU/mL but without atrial arrhythmias.
- Desiccated/natural therapy and synthetic T4+T3 were equivalent in their impact on TSH, FT3, and FT4.
- We also compared natural therapy with synthetic therapy and did not find one to be of a better value with TSH, FT3, and FT4.
- It appears, in my opinion, the main difference between T4 alone versus either combination formula was the percentage of patients who obtained free T3 in the normal range
Additional Results:
- This is a controversial subject and it appears most endocrine (America, European) bodies recommend T4 alone as the starting point/standard therapy, while The Italian Society of Endocrinology is open to combination therapy in those who have not responded to T4 alone.
- there is no consensus, even among different international guidelines. In contrast to the American Association of Clinical Endocrinologists (AACE), the European consensus group suggests that LT4 monotherapy should serve as the standard for hypothyroidism management, but they have suggested further researching LT4 and LT3 combinations in subsets of patients with symptoms (34). The Italian Society of Endocrinology does not have evidence-based data but recognizes that LT4 and LT3 therapy may be considered an experimental approach in overtly hypothyroid patients who have persistent symptoms despite adequate LT4 doses resulting in biochemical euthyroidism after exclusion of other specific causes for persistent symptoms (35).
Limitations:
- There was no control, so the placebo effect is likely making the results look more compelling than they were.
- Similarly, there is no simultaneous comparison to patients who were receiving LT4 alone
Authors Conclusion:
- Combination therapy was effective for lab value and quality of life without causing symptoms
- “the symptoms of hypothyroidism improved significantly as reported on follow-up encounters with subsequent improvement in laboratory thyroid function studies and questionnaires, and furthermore, not resulting in hyperthyroidism that caused hospitalizations for medication adverse effects, arrhythmias, or cardiac death”
- In our subset population, the symptoms of hypothyroidism improved significantly with no increase in hyperthyroidism.
- The authors also feel diligent follow up, with an experienced endocrinologist, was a reason for the success of this study
- We believe one of the reasons for our success is diligent follow-up by the endocrinologists, who see a large volume of patients with hypothyroidism
Interesting Notes:
- Classic T4 therapy is effective for 90-95% of patients, 5-10% may not optimally respond. This may be due to genetic variations that impair conversion of T4 to T3 in some
- classic monotherapy of levothyroxine (LT4) provides adequate control of symptoms for a majority of the population; however, approximately 5% to 10% of patients continue to have symptoms of hypothyroidism,
- This may occur because of dysfunctional deiodinase in a subset of hypothyroid patients
- A current meta-analysis suggests combination therapy is no more effective than standard therapy.
- “The meta-analysis of combination therapy has been negative for combination therapy in managing hypothyroidism, and the data reported on some general well-being questionnaires also are inconclusive.”
- This study ruled out confounding variables like being deficient in B12, hemoglobin and/or vitamin D
- Low levels of 25[OH] vitamin D, vitamin B12, and hemoglobin were checked in our study to rule out other causes of fatigue and hypothyroidism before starting combination therapy because they have not been ruled out in previous literature.
Clinical Takeaways:
- Combined T4+T4 therapy, either desiccated or synthetic, is a consideration for patients non-optimally responding to T4 alone.
- Follow up and monitoring to prevent overdosing may be important in these cases.
- Improvement in quality of life and TSH can be expected. T3 may also improve.
Dr. Ruscio Comments
At first glance of this abstract you might be saying ‘wow, this study seems to suggest all hypothyroid patients should be on combination therapy’. This is because it’s not clear until you read the study that this was a very specific population of subjects who were already on T4 alone and not optimally responding. This is a key distinction and reinforces the approach I have been advocating; start with T4 alone and then combination therapy is a consideration in those who have not optimally responded. Remember not to forget diet, lifestyle and gut health.
This study does provide what I feel to be important documentation of the need for some patients to be on a combination formula and hopefully will help expend practice in those resistant to this idea.
Non-celiac gluten sensitivity: All wheat attack is not celiac
https://www.ncbi.nlm.nih.gov/pubmed/29142467
Study Purpose
- “The aim of this paper is to review our current understanding of NCGS, highlighting the remaining challenges and questions which may improve its diagnosis and treatment”
Intervention:
- Review, non-systematic
Main Results:
- One of the best studies in NCGS in the AU found a 6% prevalence. A sizable number of patients experienced non-digestive symptoms
- During the period 2004 and 2010, 5896 patients were seen at the Center for Celiac Research at the University of Maryland. Of these, 347 participants met the criteria for the diagnosis of “Gluten Sensitivity” reflecting about 6% of the patients seen [12]. These gluten triggered symptoms included abdominal pain (68%), eczema and/or rash (40%); headache (35%); “foggy mind” or difficulty focusing (34%); fatigue (33%); diarrhea (33%); depression (22%); anemia (20%); numbness in the legs, arms or fingers (20%); and joint pain (11%) [12].
- Another study corroborated these non-GI findings
- Frequent symptoms reported in NCGS include bloating (87%), abdominal pain (83%), epigastric pain (52%), diarrhea (50%), and constipation (24%) [8].
- Extra-intestinal manifestations are also reported which include; lack of well-being (68%), tiredness (64%), headache (54%), anxiety (39%), “foggy mind” or difficulty focusing (38%) [8].
- Gut dysfunction seen in NCGS appears different than that of celiac
- Management of NCGS might require more than just gluten-free, including alpha-amylase/trypsin inhibitors and FODMAPS
- other food-derived stimuli have been shown to also be important in the etiopathogenesis of NCGS include alpha-amylase/trypsin inhibitors (ATIs) [26], FODMAPS [18] and other short-chain fructans.
- The role of ATIs in mounting an immunological response has been shown in animal and human research models and is believed to be an important oral antigen both in CD as well as in NCGS [26]
- Dysbiosis may also be an important aspect of treating NCGS
- The role of gut dysbiosis has also recently been emphasized in NCGS pathobiology and might help to explain both intestinal and non-intestinal manifestations in the disease by upregulating gut and systemic inflammation [3].
- Nutrient deficiencies are less common in NCGS, autoimmune conditions are less common also. Note: the autoimmune piece is important for patients peace of mind.
- Unlike CD, nutrient deficiencies such as iron, vitamin D and vitamin B 12 deficiencies are not significantly seen in NCGS [34]. NCGS also has a lesser association with autoimmune disorders when compared with CD [14,35].
- “However, we require a better understanding of the clinical presentation of NCGS, as well as its pathogenesis, epidemiology, management, and role in conditions such as irritable bowel syndrome, chronic fatigue, and autoimmunity.”
- “CD has been reported in patients with primary sclerosing cholangitis, primary biliary cholangitis, autoimmune hepatitis, aminotransferase elevations, nonalcoholic fatty liver disease, hepatitis B, hepatitis C, portal hypertension and liver cirrhosis. We evaluate recommendations for active screening for CD in patients with liver diseases, and the effect of a gluten-free diet in these different settings. Active screening for CD is recommended in patients with liver diseases, particularly in those with autoimmune disorders, steatosis in the absence of metabolic syndrome, noncirrhotic intrahepatic portal hypertension, cryptogenic cirrhosis, and in the context of liver transplantation. In hepatitis C, diagnosis of CD can be important as a relative contraindication to interferon use.”
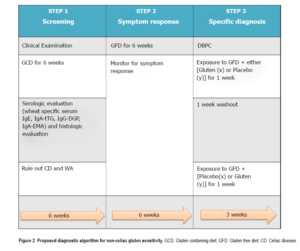
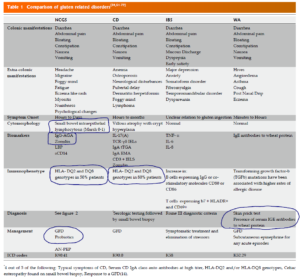
- Regarding diagnosis, the authors recommend a 6-week elimination, then followed by a 6-week reintroduction while also tracking symptoms. In those who appear NCGS, a placebo-controlled reintroduction is then recommended to confirm the diagnosis. They astutely recommend the placebo be low FODMAP. See diagram below. Note: this is not practical in a clinical setting, but is very good for research purposes.
- This symptom response is evaluated using the gastrointestinal symptom rating scale (GSRS) and a numerical rating scale (NRS). GSRS is used to identify the symptoms while the NRS is used to quantify the symptoms, weekly from week 0 (baseline) till week 6
- Symptomatic response to the GFD is defined as a decrease in 30% of the baseline score with no worsening of other symptoms in at least 50% of the observation time (3 wk) [36].
- In this step, the patient is randomly assigned into test group x or y. The patient is exposed to either GFD + placebo (x) or GFD + gluten (y) for a week. A 1-week washout period of strict GFD is observed, followed by the crossover to GFD + gluten (x) or GFD + placebo (y) [36].
- The authors suggest anti-gliadin IgG or zonulin testing. In my opinion, AGA IgG has better evidence.
- The authors also provide a helpful table to compare NCGS with other conditions
Authors Conclusion:
- “New and evolving science around gluten-related disorders have led to better understanding of the disease process, making it easier for the clinicians to set specific management guidelines. Prospective clinical trials in a diverse population are warranted to better investigate the etiology and progression of this disease. There is still a role of further research to better understand NCGS as a medical condition.”
Interesting Notes:
- “non-celiac gluten sensitivity” (NCGS) has been suggested to affect up to 6% of the United States public.”
- “the prevalence of NCGS has been reported to vary enormously from 0.6%-6% in Western populations.” [10,11,12]
Clinical Takeaways:
- NCGS may affect up to 6% of the US population.
- It may manifest with both digestive and non-digestive symptoms.
- Other dietary factors, like FODMAPs and amylase/trypsin inhibitors, may confound the association.
- Dysbiosis may be an important factor in NCGS.
- There is a lesser association with nutrient deficiencies and autoimmunity than there is with celiac disease.
- Making the diagnosis remains challenging and likely impractical in a routine clinical setting.
- AGA-IgG may be the best test but is still in the research phase.
Dr. Ruscio Comments
Not much to add here other than hopefully this summary makes it evident that more than gluten-free might be required in NCGS management.
Rapid-Fire Research: Ultra-Concise Summaries of Noteworthy Studies
The Role of a Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyol Diet in Nonceliac Gluten Sensitivity.
https://www.ncbi.nlm.nih.gov/pubmed/30158962
- Systematic review
- A low FODMAP diet has the potential for improvement of clinical symptoms in NCGS.
- In addition, some evidence suggests an additional benefit to simultaneous adherence to both low FODMAP diet and GFD.
A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects.
https://www.ncbi.nlm.nih.gov/pubmed/30048547
- Consider low FODMAP in those who react to gluten
- The low FODMAP diet was associated with a reduction in Bifidobacterium and breath hydrogen, which was reversed by oligofructose supplementation.
- The difference in breath hydrogen between groups post-intervention was 27ppm
- CONCLUSION: A low FODMAP diet reduces the total bacterial count and gas production with little effect on colonic volume.
Recommendations for treatment of hypothyroidism with levothyroxine and levotriiodothyronine: a 2016 position statement of the Italian Society of Endocrinology and the Italian Thyroid Association.
http://www.ncbi.nlm.nih.gov/pubmed/27473077
- I wanted to point out that at least one endocrine body appears open to combination T4+T3 therapy. I will review this paper in full in a subsequent issue of our newsletter. But, also remember to be cautious and not overuse T3 as appear common practice in functional medicine.
- Levothyroxine (L-T4) is recommended as lifelong replacement therapy for hypothyroidism. Recent clinical and experimental data support the addition of levotriiodothyronine (L-T3) treatment in some selected hypothyroid patients when their symptoms persist and their quality of life remains impaired despite adequate L-T4 monotherapy.
- An increase in L-T3 prescriptions has been recently observed in Italy due to the availability of different L-T3 formulations, making it possible to clinicians to prescribe L-T3 alone or in combination with L-T4.
- The aim of the present position statement was to define the correct clinical indications, schedule, duration of treatment and contraindications of combined treatment with L-T4 and L-T3 in hypothyroid patients in an attempt to guide clinicians and to avoid potential adverse effects of overtreatment.
Effectiveness and safety of calcium and vitamin D treatment for postmenopausal osteoporosis.
https://www.ncbi.nlm.nih.gov/pubmed/26205648
- The jury is still out, according to this paper, on the safety of calcium supplementation.
- At the moment it is not possible either to provide reassurance that calcium supplements given with vitamin D do not cause adverse cardiovascular events or to link them with certainty to increased cardiovascular risk.
- According to the data now available, vitamin D, at a dosage of at least 800 IU/day, alone or in combination with antiresorptive drugs, should be administered in osteoporotic and osteopenic patients for primary and secondary prevention.
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next practitioner question of the month.

Like what your reading?
Please share this with a colleague and help us improve functional medicine
Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!