Practitioner Research Review – September 2018
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
In Today’s Issue
Research
- Non-celiac gluten sensitivity: people without celiac disease avoiding gluten—is it due to histamine intolerance?
- Serum-Derived Bovine Immunoglobulin/Protein Isolate Therapy for Patients with Refractory Irritable Bowel Syndrome.
- A Review of the Use of Biotin for Hair Loss.
- Rapid-Fire Research – ultra-concise summaries of noteworthy studies
- Serial Frozen Fecal Microbiota Transplantation in the Treatment of Chronic Intestinal Pseudo-Obstruction: A Preliminary Study
- Escherichia coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-Analysis
- Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis
- Iodine Nutrition Status and Thyroid Disorders: A Cross-Sectional Study From the Xinjiang Autonomous Region of China
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however, the research studies are a collection of the most clinically meaningful research that has been published recently.
Non-celiac gluten sensitivity: people without celiac disease avoiding gluten—is it due to histamine intolerance?
https://www.ncbi.nlm.nih.gov/pubmed/29181545
Study purpose
- To review the similarities between non-celiac gluten sensitivity (NCGS) and histamine intolerance (HIT)
Intervention:
- Review, non-systematic
Main Results:
- There is a large overlap between the symptoms of NCGS and histamine intolerance
- “we show that intestinal and extra-intestinal NCGS symptoms are very similar to those which can be found in histamine intolerance.”
- Specifically, there are similar digestive symptoms in both NCGS and HIT
- Gastrointestinal nonspecific symptoms in HIT include postprandial fullness, flatulence, bloating, abdominal pain, loose stools, diarrhea and/or constipation.
- And similar extra-intestinal symptoms
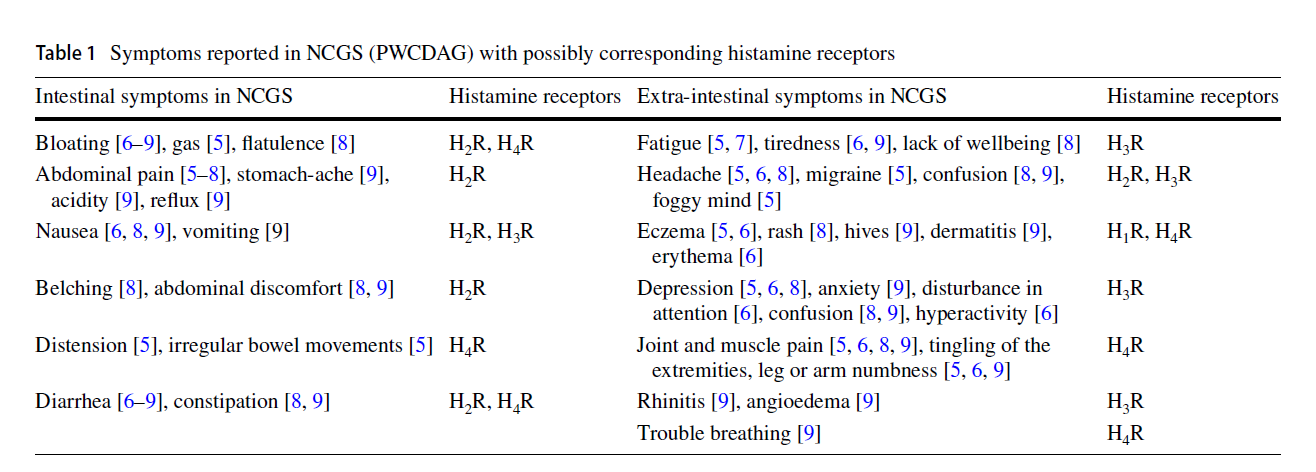
- Extra-intestinal symptoms include headache, migraine [27], foggy mind, chronic fatigue [28], joint and muscle pain, tingling of extremities, leg or arm numbness [29], eczema [30], asthma [31] and depression [28].
- The authors summarize this into a table with the corresponding histamine receptors:
- Anemia may be the only symptom unique to NCGS
- The only symptom which is described with NCGS (PWCDAG) that was not included in the table of symptoms (Table 1) is anaemia, because there is no correlation to histamine receptors
Additional Results:
- Increased dietary intake combined with a poor ability to metabolize histamine is what causes histamine intolerance.
- A disproportionate amount of histamine in the body is suspected to result from the consumption of histamine-containing food or drinks, and a reduced ability of enzymes (diamine oxidase and histamine N-methyl transferase) to digest histamine.
- Damage to the small intestinal lining reduces histamine metabolism.
- Mucosal damage in the small intestine caused by e.g., gastroenteritis, short bowel syndrome, gastrointestinal surgery and various drugs may also reduce DAO activity
- DAO is the primary enzyme in the gut which metabolizes histamine. Note: I do not find supplementing with this enzyme necessary for most nor is it cost-effective.
- Histamine is widely distributed throughout the body and within the gastrointestinal tract DAO appears to be the primary enzyme for the degradation of ingested histamine
- However, it is not all about the small intestine. Some HIT might be genetically determined.
- There are polymorphisms identified in the genes coding for DAO [39, 40] and for the known histamine receptors [41] which may help to explain the wide individual variability of symptoms observed in multiple organs.
- Definitive testing has not been established. However, serum DAO enzyme levels <10 has been suggested. HOWEVER, the lab finding requires the corresponding clinical context of GI symptomatology plus response to low histamine diet to be diagnostic.
- Standardized in vitro diagnostic tests for HIT testing are still lacking, but the diagnosis of HIT may be supported with the measurement of diamine oxidase in serum [42].
- Although serum DAO values have not been shown to correlate with gastrointestinal DAO activity, patients with reduced serum DAO activity (< 10 U/mL), two or more typical gastrointestinal symptoms described for HIT, and a reduction of abdominal complaints after following a histamine reduced diet, may be diagnosed with HIT
- Histamine intolerance might be more prevalent in those with carbohydrate malabsorption.
- Recently a high prevalence of low serum DAO values in patients with carbohydrate (lactose and fructose) intolerance/malabsorption was described. In patients with non-specific gastrointestinal symptoms seven combinations of intolerance/malabsorption were described and more than 55% of these patients demonstrated DAO values < 10 U/ml
Authors Conclusion:
- “After a detailed diagnostic workup for all possible etiologic factors in every patient, a targeted dietary intervention for single or possibly combined intolerance/malabsorption might be more effective than a short-term diet low in fermentable oligo-, di- and monosaccharides and polyols (FODMAP) or the untargeted uncritical use of gluten-free diets.”
Clinical Takeaways:
- Histamine intolerance might be responsible for presumed NCGS
- Histamine intolerance is more common when small intestinal health is suboptimal
- Histamine intolerance is more common in those with carb malabsorption
Dr. Ruscio Comments
Could some patients who believe themselves to be NCGS not be gluten sensitive? Yes! Two things to look out for are FODMAP intolerance and histamine intolerance. This is important to know because by default patients will usually revert to an overly restrictive relationship with gluten due to hyper-avoidance ethos permeating many niches in healthcare.
It is my thinking that either a low FODMAP diet or a low histamine diet can repair the intestines and improve histamine metabolism. I draw this inference based on the fact that a low FODMAP diet leads to an 8-fold decrease in histamine levels (perhaps due to improved SI health). And because one study has shown increased DAO enzyme production after a low histamine diet (study to be detailed in a future issue of the FFMR). So, patients can heal and recover from histamine intolerance, I routinely see this in the clinic.
Serum-Derived Bovine Immunoglobulin/Protein Isolate Therapy for Patients with Refractory Irritable Bowel Syndrome
http://www.scirp.org/journal/PaperInformation.aspx?paperID=50770
Study Purpose
- Assess the effect of serum-derived bovine immunoglobulin/protein isolate (SBI) for those with non-responsive IBS.
Intervention:
- A retrospective review of 35 IBS patients with diarrhea or mixed diarrhea/constipation pattern (IBS-M) who were administered SBI 5 grams twice daily for 4 weeks was performed.
- All patients were non-responsive to prior IBS therapies.
- This study included IBS patients with and without SIBO.
- Therapeutic sequence: All patients were assessed for SIBO. Those with SIBO were treated with antibiotics and then offered standard IBS therapies. Those without SIBO were offered standard IBS therapies. Standard IBS therapies included: low FODMAP, Loperamide (Imodium), probiotics, anti-depressants
- Before being placed on SBI, all patients with a positive LBT were first placed on a 14-day course of 550 mg rifaximin three times per day (TID) with or without metronidazole or neomycin. Many of these patients were then placed on a variety of other common IBS treatments, including tricyclic anti-depressants, loperamide, anti-cholinergics and diet modification (FODMAP). If there was an incomplete response after these therapies, then they were prescribed SBI as a nutritional standard-of-care.
- All patients who initially had a negative LBT and who had failed conventional IBS treatments to sufficiently manage their condition as determined by the clinician (FODMAP diet, probiotics, tricyclic anti-depressants and/or alosetron) were placed on 5 grams SBI BID for four weeks without other therapies
Main Results:
- Overall (pooled) response rate was 75% and was statistically significant. Pooled meaning includes SIBO positive and SIBO negative subjects.
- Adverse events were reported in 8% of cases (3 patients). 1 patient reported constipation, 1 diarrhea, and 1 nausea. Event dissipated after stopping the treatment.
- The SIBO positive subgroup’s response rate was not statistically significant (the p-value was >0.05).
- The symptoms that constituted a ‘response’ were improved regularity, abdominal discomfort, abdominal bloating and flatulence/lower gas.
Additional Results:
- What is the mechanism of SBI?
- Binding of toxins in the gut, which may have downstream effects of barrier healing
- Serum-derived bovine immunoglobulin/protein isolate (SBI), a FDA-regulated medical food product, is composed of greater than 50% immunoglobulin and has been shown to survive the stomach environment and bind microbial components in the intestine, thereby neutralizing their effects in many different animal models
- The microbial binding activity of SBI may have downstream effects in maintaining GI immune balance and managing gut barrier function
- Binding of toxins in the gut, which may have downstream effects of barrier healing
- Have RCTs shown benefit? Yes.
- In a double-blind, randomized, placebo-controlled IBS-diarrhea (IBS-D) study, 10 grams of SBI per day were shown to cause a statistically significant decrease the number of days per week that patients experience—abdominal pain, flatulence, urgency, loose stools, bloating or any symptom [1].
Limitations:
- There are some important limitations here. The survey used to assess patient symptoms was non-validated. A non-validated questionnaire may diminish the strength of these findings.
- There was no control group. We know in randomized-control IBS studies the placebo effect averages 45%, so perhaps it would be more accurate to roughly estimate the response rate as 75% (as found in this study) minus 45% (the likely placebo effect) = estimated total response rate of 30%.
Authors Conclusion:
- “SBI appeared to be a safe and effective nutritional moiety in refractory IBS-D and IBS-M patients. Larger, double-blind studies are needed.”
Clinical Takeaways:
- SBI is a nutritional therapy that may help otherwise non-responsive IBS cases.
- It is unclear whether those with IBS and who are SIBO+ will also benefit.
Dr. Ruscio Comments
SBI is the most exciting new therapy for IBS I have come across in the past few years. Yes, there are lots of therapies out there which ‘talk a good game’ but then when I look into the evidence there isn’t much there. Fortunately, for SBI there has been a handful of clinical trials (3, perhaps a 4th) demonstrating improvements in clinical endpoints. I will summarize another paper or two on SBI in the coming months. This is available over the counter as SBI Protect or via prescription as EnteraGam.
A Review of the Use of Biotin for Hair Loss
https://www.ncbi.nlm.nih.gov/pubmed/28879195
Study Purpose
- Review biotin’s effect on hair loss
Intervention:
- Systematic review
Main Results:
- Biotin appears to improve hair loss but in those with an underlying pathology present. Evidence substantiating improvement in healthy/normal individuals is lacking.
- We found 18 reported cases of biotin use for hair and nail changes. In all cases, patients receiving biotin supplementation had an underlying pathology for poor hair or nail growth. All cases showed evidence of clinical improvement after receiving biotin
- However, we propose these cases are uncommon and that there is lack of sufficient evidence for supplementation in healthy individuals.
- Despite these data, there have been no randomized, controlled trials to prove the efficacy of supplementation with biotin in normal, healthy individuals.
Additional Results:
- Most people are meeting their daily requirements for biotin of 30 µg/day.
- Current recommendations for biotin by the Institute of Medicine state that the daily adequate intake (AI) for adults is 30 μg/day [1]. Most healthy individuals meet these requirements through a well-balanced diet, though many still take up to 500–1,000 μg of biotin supplementation daily.
- reports of low biotin levels have rarely been cited
-
Foods high in biotin include nuts, legumes, whole grains, unpolished rice, and egg yolk
- Symptoms of biotin deficiency “whether acquired or congenital, typical symptoms of biotin deficiency include alopecia, eczematous skin rashes, seborrheic dermatitis, conjunctivitis, and multiple neurological symptoms, such as depression, lethargy, hypotonia, and seizures”
- Testing can be challenging but is possible via urinary metabolites
- The normal biotin plasma concentration ranges from 400 to 1,200 ng/L [22] . Deficiency is technically considered to be a level of less than 200 ng/L. However, plasma biotin levels can fluctuate daily and thus are not considered to be a sensitive marker [22]. A more validated measure of biotin deficiency is an increased urinary excretion of the metabolite, 3-hydroxyisovaleric acid (normal level: 195 μmol/24 h)
- One study has suggested that anywhere from 27-38% of those with hair loss might have a biotin deficiency. So, it is possible more research could document benefit from supplementation.
- Moreover, only 1 case in the literature has measured the levels of biotin in normal individuals that had complaints of hair loss. In this study of 541 women (age range between 9 and 92 years), 38% had low biotin levels [20]. However, of those women, 11% were later found through patient history (use of antibiotics, antiepileptics, isotretinoin, or GI disease) to have a reason for the underlying deficiency and 35% had co-existing seborrheic dermatitis, suggesting a multifactorial cause for hair loss.
Authors Conclusion:
- Despite its popularity in the media and amongst consumers, biotin has no proven efficacy in hair and nail growth of healthy individuals. Only 1 study has shown decreased levels of biotin in healthy individuals, though this data was confounded by multiple factors, including patient history. Therefore, in the absence of additional studies, we have found no evidence to suggest benefit from biotin supplementation outside of known deficiencies secondary to congenital or acquired causes.
Clinical Takeaways:
- There is no good evidence to support biotin supplementation for hair loss.
- Most people are biotin sufficient.
- A short trial may be justifiable based upon one study’s results.
Dr. Ruscio Comments
I have often heard of biotin as a ‘go to’ supplement for hair loss. However, knowing there are many false truths circulating in natural medicine, I wanted to see if there was any evidence to support this recommendation. Overall it appears there is no good evidence, but it might be justifiable to perform a trial on biotin for 3-5 months and continue only if a clear benefit is noted. Those with GI malabsorption might have an increased probability of benefiting from this.
Rapid-Fire Research – Ultra-Concise Summaries of Noteworthy Studies
Serial Frozen Fecal Microbiota Transplantation in the Treatment of Chronic Intestinal Pseudo-Obstruction: A Preliminary Study
https://www.ncbi.nlm.nih.gov/pubmed/27840368
- This was a small study, in a unique population, but it did show that FMT can eradicate SIBO in roughly 70% of cases while also improving symptoms.
- SIBO was eliminated in 71.0% (5/7) of patients
- FMT significantly alleviated bloating symptoms, and symptoms of pain were relieved 2 weeks after FMT
- I would only reserve FMT as a consideration for those who are still ill after exhausting all other options. These findings do partially illustrate a concept some of the SIBO community seems slow to embrace, you can fight bacteria with bacteria.
- Also, FMT may reduce transit time [4]
Escherichia coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-Analysis
https://www.ncbi.nlm.nih.gov/pubmed/26697577
- E. coli Nissle was found to be equivalent to Mesalazine in maintaining remission in Ulcerative colitis. E. coli Nissle cannot yet be recommended to induce remission, however.
- EcN is equivalent to mesalazine in preventing disease relapse, thus confirming current guideline recommendations.
- EcN seems to be as effective as controls in inducing remission and therefore, its use cannot be recommended as in one study the comparison was performed against placebo.
Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis
https://www.ncbi.nlm.nih.gov/pubmed/27702392
- We have briefly touched on this study’s results previously. Selenium was shown to reduce thyroid antibodies after up to 3 months of use in those not on thyroid hormone.
- Selenium was shown to reduce thyroid antibodies after up to 12 months of use in those on thyroid hormone.
- Selenium supplementation reduced serum TPOAb levels after 3, 6, and 12 months in an LT4-treated AIT population, and after three months in an untreated AIT population. Whether these effects correlate with clinically relevant measures remains to be demonstrated.
- The group distinction is important because thyroid hormone can reduce antibodies so the longer term ‘effect’ of selenium in those also on thyroid hormone might be due to the hormone.
Iodine Nutrition Status and Thyroid Disorders: A Cross-Sectional Study from the Xinjiang Autonomous Region of China.
https://www.ncbi.nlm.nih.gov/pubmed/27188916
- While we have covered much data showing the harmful effects of iodine, it is important to mention that deficiency is also problematic.
- Thyroid disorders, especially hyper/hypothyroidism and subclinical hypothyroidism, are more likely to be prevalent in an iodine-deficient population.
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next practitioner question of the month.

Like what your reading?
Please share this with a colleague and help us improve functional medicine

Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!