Practitioner Research Review – January 2017
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however the research studies are a collection of the most clinically meaningful research that has been published recently.
Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome.
https://www.ncbi.nlm.nih.gov/pubmed/27747984
Study purpose:
- To evaluate the effect of a low FODMAP diet compared to a diet high in prebiotics on IBS symptoms and related co-morbidities.
- Markers tracked
Intervention (double blinded):
- 20 patients with IBS (IBS-D and IBS-M) followed a low FODMAP (LFD) diet for 3 weeks, then
- ½ patients received 16g/day of FOS prebiotics, the other ½ received a placebo as maltodextrin
- Then all patients performed another 3 weeks on low FODMAP
- Then the groups were switched – ½ patients reviewed FOS and the other ½ received maltodextrin
Results:
- “There was a significant improvement in all IBS symptoms after 3 weeks of LFD”
- “…an overall patient satisfaction of 85%” was reported on the low FODMAP diet
- Those on the low FODMAP diet also experienced a significant improvement in
- When then transitioning to either placebo or FOS
- When on FOS, patients experienced a worsening of
Additional results:
- Cytokines: A reduction in inflammatory cytokines was noted in those on the low FODMAP diet, but not for those on FOS. Specifically IL-6 and IL-8.
- Microbiota: Paradoxically, patients became slightly more dysbiotic while on low FODMAP but then slightly less dysbiotic when on FOS. This included a reduction of Bifidobacterium.
- SCFAs: low FODMAP caused a slight reduction
Authors’ Conclusion:
- “Our findings support the efficacy of a LFD in alleviating IBS symptoms, and show changes in inflammatory cytokines, microbiota profile, and SCFAs, which may have consequences for gut health.”
Discussion:
- No specific bacterial changes appeared to correlate with cytokines, SCFAs, or symptoms.
- Bifidobacterium paradox: Bifidobacterium decreased on the low FODMAP diet when symptoms improved, then Bifido increased on FOS when symptoms flared. However, many trials have shown Bifidobacterium probiotics to be one of the most effective probiotics for IBS.
- A few other studies have also showed decreased immune activation on low FODMAP diet, including reductions in histamine. (“Proinflammatory cytokines and histamine are both synthesized and secreted by inflammatory cells”).
- The authors also make an important note that the microbiota rapidly changes based upon diet and that any bacterial loss on low FODMAP could easily be corrected by changing one’s diet i.e. low FODMAP will not cause you to lose bacteria permanently.
Clinical Takeaways:
- The low FODMAP diet is a highly effective intervention for IBS. Prebiotics should be used with caution in IBS.
Dr. Ruscio’s Comments
When we step back and look at all of this, what does it mean? How do we interpret the fact that as symptoms improved and inflammation decreases, we also see a loss of bacteria (including “good bacteria” like Bifidobacterium), a decrease in SCFAs, and patients seem to become more dysbiotic? My thinking is that those with IBS and IBD have an exaggerated immune response against their commensal GI bacteria i.e. their microbiotas. When we feed what the immune system is attacking, we see increased symptoms and inflammation. It might be that, in part, the dysbiosis seen in IBS and IBD is occurring secondary to the overzealous immune system – meaning it’s an adaptation to protect the host. In any case, we can prevent ourselves from making poor clinical decisions if we follow the clinic outcome studies and avoid speculating as to what a good treatment might be based upon observation or mechanism. In this case, while the exact mechanism may be confusing, it’s clear that the low FODMAP diet is effective clinically while prebiotics appear detrimental clinically.
Anti-Thyroperoxidase Antibody Levels >500 IU/ml Indicate a Moderately Increased Risk for Developing Hypothyroidism in Autoimmune Thyroiditis.
http://www.ncbi.nlm.nih.gov/pubmed/27607246
Study purpose:
- “to investigate the association between thyroid antibody levels and the risk for developing hypothyroidism” – essentially to see if thyroid autoimmunity predicts future hypothyroidism.
- TPO and Tg antibodies (thyroperoxidase and thyroglobulin) were assessed in correlation with hypothyroidism (TSH).
Intervention:
- 335 patients were assessed for correlation between thyroid antibodies and hypothyroidism
- 21 of these patients underwent long term monitoring to track association between thyroid autoimmunity and later development of hypothyroidism
Main Results:
Correlation between thyroid antibodies and hypothyroidism
- TPO or Tg antibodies <100 and between 100–500 had no significant different TSH levels.
- TPO >500 was significantly associated with moderate risk for elevations in TSH.
- If TPO AND Tg antibodies were each both above 500, it was also significantly associated with increased elevations in TSH.
- Tg antibodies alone >500 did increase risk elevated TSH but not significantly.
- Dr. R’s Note: this means TPO may be more important in predicting hypothyroidism.
Correlation between thyroid antibodies and future hypothyroidism
- 21 of the 335 patients were tracked for an average of 6.2 years to assess the association between thyroid antibodies and future development of hypothyroid
- No progression toward hypothyroidism was seen for 29% (6/21) of patients
- 71 % (15/21) patients showed progression toward hypothyroidism
- – Progression defined as an increase of the TSH level over the time
- – However, even though the increase was a statistically significant increase in those with TPO >500, it did not appear to be clinically meaningful. The average TSH increase was 0.5 per 6.2 years
- – Which explains why “almost all patients in this group stayed in the euthyroid stage (TSH < 4.6” – meaning almost all patients that progressed toward hypothyroid did not actually become hypothyroid.
- – Only one patient became truly hypothyroid after 8.2 years of follow up.
Authors’ Conclusion:
- “Our data indicate largely elevated levels of TPO-Abs being associated with a moderately increased risk of developing hypothyroidism.”
Interesting Notes:
- “Clinically, AIT is among the most common human autoimmune disease with an annual incidence worldwide.”
- Other studies have examined the relationship between thyroid autoimmunity and hypothyroid but this is the first one to assess if the magnitude of autoimmunity impacts the association.
Clinical Takeaways:
- Keeping TPO antibodies below 500 may decrease ones chance of future progression to hypothyroid.
- Tg antibodies appear to have less predictive utility than TPO.
Dr. Ruscio’s Comments
With this study, we make a case for interventions that lower antibodies – this is speculative but reasonable. Also, this information creates a realistic expectation for what pathological versus ‘non-pathological’ levels of antibodies are. For example, I have said that in my observation TPO antibodies between 100-300 are a ‘clinical win’ and that Tg doesn’t appear to be have a clear association. This study reinforces that and expends what a minimal risk level of TPO might be to below 500.
This is an important educating point for your patients because many patients are fearful that any elevation of antibodies is highly pathological. This can create stress and fear and cause patients to pursue more elaborate treatments when they may not be justified. In fact, according to this study’s results, we could speculate that the fear instilled by a clinician mismanaging this conversation could be worse than the actual disease itself. So remember, if someone is feeling well and their TPO antibodies are below 500 they are likely in good shape. Also, remember that this study found that even if someone is above 500 they will only experience roughly a 0.5 elevation in antibodies every 6.2 years. This should be reassuring the patients and clinicians alike.
Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study
http://www.ncbi.nlm.nih.gov/pubmed/27444264
Study purpose:
- To assess which pathway is more influential – the brain-gut connection or the gut-brain connection.
Intervention:
- 1,900 people were prospectively tracked for 1 year to assess if psychological symptoms appeared before GI symptoms or vice versa. Anxiety, depression, IBS, and general quality of life were tracked.
Main Results:
- In 1/3rd of subjects psychological symptoms appeared before GI symptoms
- In 2/3rd of subjects GI symptoms appeared before psychological symptoms
Authors’ Conclusion:
- “While brain–gut pathways are bidirectional, a major subset begin with gut symptoms first and only then psychological distress develops, implicating primary gut mechanisms as drivers of the gut and extra-intestinal features in many cases.”
Discussion:
- “These data suggest low grade gut inflammation in IBS and FD (e.g. via mast cell or eosinophil infiltration) with cytokine release may at least in a subset directly induce the observed psychological comorbidity so deeply characteristic of the syndromes.”
- “the gut likely drives psychological alterations in a major group of cases with FGIDs (functional gastro-intestinal disorders).”
- Not all data agree with the gut-brain connection being more common, http://www.gastrojournal.org/article/S0016-5085(12)62189-1/abstract
- “A new model of a microbe-gene-inflammation interaction, where infection can lead to excessive tumour necrosis factor alpha and other cytokine production in vulnerable individuals is beginning to emerge and may in part explain this gut–brain interaction.”
- “…those whose FGID symptoms that arise primarily from gut pathways will not respond well to psychological therapy, but will respond to gut directed therapies that reverse key abnormalities such as an abnormal microbiome.”
Clinical Takeaways:
- In those with both GI symptoms and anxiety/depression – start with the gut and then reevaluate.
Dr. Ruscio’s Comments
The gut and brain share a bi-directional relationship. Clinically, the question often arises where to start first – the gut or the brain? Much of this will depend on what you find in history and examination, however this study does suggest that starting with the gut may be more important for the majority. It’s also important to remember that not all GI disorders manifest as GI symptoms, so it is possible that if someone had only anxiety/depression (or other neurological symptoms) there still may be a silent GI issue driving this.
Predicting a Response to Antibiotics in Patients with the Irritable Bowel Syndrome
https://www.ncbi.nlm.nih.gov/pubmed/26362282
Study purpose:
- To assess if SIBO breath test results predicted response to antibiotic treatment.
Intervention:
- 561 patients with Rome II diagnosed IBS performed a lactulose breath test. Hydrogen and methane were assessed every 15 minutes for 3.5 hours
- A variety of antibiotics were used, including rifaximin, amoxicillin/clavulanic acid, levofloxacin, doxycycline, ciprofloxacin, neomycin, or metronidazole.
- “A positive response to antibiotics was defined as an improvement in the patient’s global IBS symptoms of at least 50 %”
Main Results:
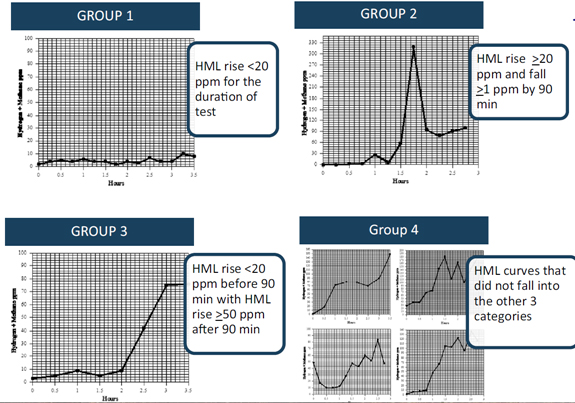
-
- Test results were broken down into 4 SIBO test presentations
-
- – (Group 1) those whose HML did not increase by >20 ppm throughout the duration of the test
- – (Group 2) those with an HML rise >20 ppm with a drop of at least 1 ppm after the initial rise but before 90 min
- – (Group 3) those whose HML did not increase by >20 ppm by 90 min but subsequently increased by > 50 ppm
- – (Group 4) those graphs that do not fall in one of the aforementioned categories
- Antibiotic response according to group:
- – Group 1 = 94.7 % 175
- – Group 2 = 81.4 %
- – Group 3 = 47.2 %
- – Group 4 = 79.9 %
- “… the fact that absence of any rise in gases (group 1) predicted the best response (94.7 % improvement rate) is an unexpected, novel observation.”
Additional Results:
- Ciprofloxacin was most effective treatment, followed by Rifaximin + Neomycin.
Authors’ Conclusion:
- “A lactulose breath test appears to be useful in predicting response to antibiotics in patients with the irritable bowel syndrome. A hydrogen + methane rise <20 ppm throughout the duration of the test is most predictive. This observation contradicts the classic definition of a positive lactulose breath test.” Note: strongest response was in group 1.
Interesting Notes:
- “Antibiotics for presumed small intestinal bacterial overgrowth have been shown to improve irritable bowel syndrome symptoms in at least 40 % of subjects.”
- “About 15 % of the population of North America are thought to suffer from IBS.”
- “… when studies attempted to compare differences in breath tests between patients with IBS and healthy controls, results were disappointing . This is possibly why, when Pimental et al. reported the results of a large multicenter study using rifaximin to treat patients with IBS, breath testing was not used to select patients.”
Clinical Takeaways:
- Those without SIBO may respond symptomatically to antibiotics (or antimicrobials). Those with classical SIBO may also respond. However, those with a ‘false positive’ presentation (group 3) may respond poorly to treatment.
Dr. Ruscio’s Comments
Perhaps the reason why lower gas levels (Group 1) predict a better response to one round of treatment is because if someone has higher gas levels then more than one round of treatment would be needed. This was suggested in another recent study: https://drruscio.com/how-long-should-you-treat-sibo/ .
It’s also important to mention that Group 2, which is more of a classical SIBO presentation, did respond well (81.4%). Additionally, Group 3 responded much less (47.2%), and this is likely because this pattern is more representative of a false + with SIBO. https://drruscio.com/what-is-the-best-test-for-sibo-lactulose-or-glucose-breath-testing/
With all this said, remember that SIBO testing is not the end-all-be-all for IBS treatment. Practical treatment monitoring will likely obtain the best results.
Hypothyroid symptoms and the likelihood of overt thyroid failure: a population-based case–control study
https://www.ncbi.nlm.nih.gov/pubmed/25305308
Study purpose:
- To determine what symptoms are most closely associated with autoimmune hypothyroid.
Intervention:
- Symptoms were compared in new overt autoimmune hypothyroidism (n=140) and thyroid disease-free controls (n=560)
Main Results:
- Hypothyroid patients suffered mostly from
- – Tiredness (81%), dry skin (63%), shortness of breath (51%), hair loss (30%)
- “…we identified 13 ‘hypothyroidism-associated symptoms’:
- – globulus sensation, difficulty swallowing, anterior neck pain,
- – wheezing, shortness of breath, palpitations,
- – constipation, hair loss, dry skin, restlessness,
- – mood lability, tiredness, and vertigo.”
- Reporting 3 or more of these symptoms was significantly associated with autoimmune hypothyroid
- – If fatigue is not present than hypothyroidism is unlikely
- – If dry skin is not present than hypothyroidism is unlikely
Additional Results:
- Average Case Result
- TSH = 54.5
- TPO = 4588
- Tg = 131
- Average Healthy Control Result
- TSH = 1.24
- TPO = <30
- Tg = <20
Authors’ Conclusion:
- Paraphrased – symptoms alone do not adequately predict hypothyroidism, so any suspicion should prompt a blood test.
Interesting Notes:
- This study did not assess every possible symptom that could be associated with hypothyroidism, for example, cold intolerance and less sweating were not assessed.
- The overall predictive value of symptoms is low.
Clinical Takeaways:
- Based upon these findings, we can sharpen our intake paperwork’s thyroid section, but any suspicion should be verified by a blood test.
Dr. Ruscio’s Comments
If you would like to sharpen your intake paperwork, you could use these 13 symptoms as your thyroid assessment. I would list fatigue and dry skin first, and be cognizant that the presence of one or both of these two symptoms combined with checking 3 or more total symptoms is a significant predictor.
It’s also interesting to look at the lab values here. TSH was, on average, frankly elevated in these patients. The TPO antibodies were also much higher than the 500 cut-off we discussed in the previous study – which makes sense. However, and as we discussed in the previous study also, Tg antibodies did not seem to correlated with autoimmune hypothyroid.
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next practitioner question of the month.

Like what your reading?
Please share this with a colleague and help us improve functional medicine
Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!