Dr. Ruscio’s Microbiota Presentation
Dr. Ruscio presented on The Gut; Diet, Flora, Health and Disease at the Ancestral Health Society’s 2014 symposium at UC Berkeley. He reviews the impact hygiene has on our health, hunter gather versus westernized microbiotas and what the science actually says about manipulating our microbiotas with probiotics, prebiotics and fiber.
The Gut Microbiota; Clinical Pearls Vs. Marketing Ploys & Regaining Your Ancestral Gut
I’d like to introduce Dr. Michael Ruscio, who will speak about the gut microbiota in health and disease, research, and recommendations.
Dr. Michael Ruscio: Thank you. Well, hi, everyone. Thank you for coming. Can everybody hear me OK? OK. My name is Michael Ruscio. I am an alternative physician here in Walnut Creek. Gastrointestinal problems are a major part of my practice, and they were a major part of my own career. I was going into conventional medicine and then I ended up acquiring an amebic infection. It really opened up my eyes (to) how important the gut was, and I shifted gears into alternative medicine. And so now, pretty much every patient I see, we have some kind of gut work that we have to do. I have been really following the microbiota research to see if we can extract any kind of clinical pearls from that. So, we’ll jump right in there. There’s a lot of stuff that I want to cover.
We will talk about what is the microbiota, and why is it important?
We will look at a comparison of different microbiotas – modern versus ancestral; and also westernized versus developing areas. We will look at the microbiota in disease IVD, obesity and autoimmunity.

And we’ll also look at manipulating the microbiota, with diets, probiotics, prebiotics, fiber, and antimicrobials. There are other things here that we could potentially put in, like fecal transplant and helminthic therapy, but those aren’t really accessible to many people. So, I’m going to focus of the things that you have more readily available to you. And then we’ll wrap up with a few conclusions.
So, to quote:
“The gut microbiota has coevolved with humans and can be considered an organ of similar size as the liver. The relationship between the microbiota and the host is one of the most ancient stories of coevolution.”

In fact, as we look at different populations we see different bacteria that shift in order for that population to survive. One of the things that we can’t do as humans is evolve our genes very quickly. So, if the environment changes rapidly, we’re in trouble, because we can’t always change our DNA and our genes to keep pace with that. But we can borrow from bacteria that have the ability to very rapidly evolve their DNA through what’s known as horizontal gene transfer. One of the things that’s happened is as the environment has changed quickly, and we haven’t necessarily been able to change our genes to keep up, we’ve borrowed the fast, adaptable genes of bacteria to allow us to keep pace with the changing environment.
OK, so what is the microbiota and why is it important?

Well, it does a number of things for us – Digestion: Roughly 10 percent of our calories are from bacterial fermentation, production of B vitamins and K vitamins, and what we just discussed; it enables us to survive and adapt faster using bacterial genes. And we also see the production of short chain fatty acids, which are reparative to the intestinal cells. We also see toning of the immune system and the ability to crowd out pathogens from being able to infect the GI tract. Detoxification, breakdown and removal of various toxins is also assisted by the gut microbiota and also a partial regulation of metabolism.
So, when you read some of the literature, it can be very confusing. Sometimes they talk in phyla, sometimes they talk in genus, sometimes they talk in species. So, I just want to give a quick overview to help orient you to some of the nomenclature.
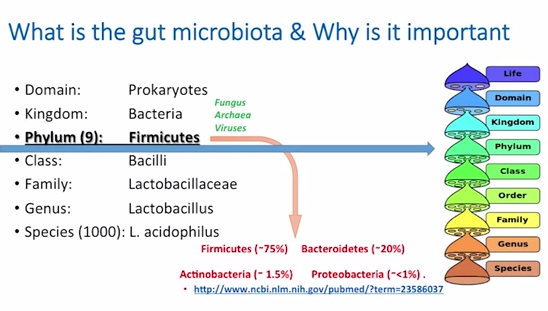
So, the domain is Prokaryotes; the kingdom is, of course, Bacteria. But there are also some fungus – people have probably heard of candida infections, and yeast, and things like that. There is also Archaea – the most well-known is mathanobraver smithii, which is the organism that can cause constipative small intestinal bacterial overgrowth; it’s a form of organism that secretes methane gas, and you’ll see this on testing for people who have small intestinal bacterial overgrowth. And also, interestingly, viruses play a role in the gut. Now, what bacteria will do is they will use viruses to exert antibacterial effect over some of the other bacterial competitors through what’s known as phage function. So, viruses are also there; they are a small percent, but viruses also play a role in the microbiota
Now, at the Phylum level, there are about nine well-established phylum, one of which is Firmicutes. This is where we currently have the best level of understanding. We go deeper into class, family, genus, like Lactobacillus, and species – lactobacillus acidophilus, of which there are about 1,000. We usually speak in terms of lactobacillus acidophilus, bifidobacterium infantis – this is what we are typically used to speaking about. But, (in) the literature, we see much more of an understanding here sometimes with species mix in, because we just haven’t gotten good enough to get that deeper level of understanding down to the species level yet.
When we look at the phylum, we see Firmicutes is usually the major player in pretty much every society that we see and in most mammals in general. Bacteriodetes is usually second to that, and Anctinobacteria and Proteobacteria represent more of a minority. And these links that you are seeing here are just to the PubMed abstracts if you wanted to pull the papers on these.
OK, so now lets get into comparison of different microbiota.
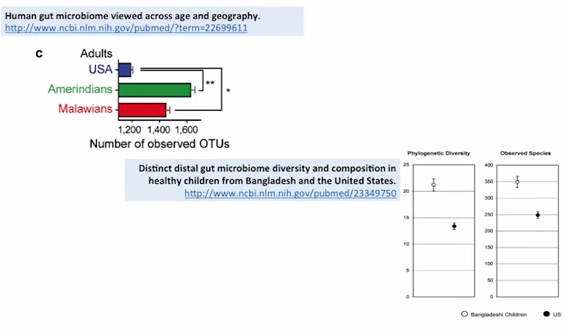
So I what to give you a summary of this section: Increase diversity seen with decreased hygiene. So, let’s talk about that briefly. So, in this study you’re looking at the number of operational taxonomical units. This is just a measure of diversity. So the more of that you see – the more diverse bacteria that you have, just good, that’s what you want, diversity is good – so you see the US pales in comparison to these other two non-industrialized, non-highly hygienic societies, the Amerindians and the Malawians.
So you see, when we compare more Third World-type countries, they have much more bacterial diversity in their microbiota. Again, the same thing represented in another study, where the compared the gut of children from the United States to those of Bangladesh. And we see here both a phylum-level comparison and a species-level comparison – Bangladesh are the empty circles, (while) the US are the solid circles. You see againfar more diversity in the Bangladesh children. OK, so the dirtier the environment, the more diverse a colonization.

So now, No. 2: Novel, even ‘pathogenic’ bacteria, may be present in certain areas to aid with digestion of unique foodstuffs. If we look at the Hadza – The Hadza are maybe the last group of untouched hunter-gatherers. This is the are where they habitate. They have 70 percent of their diet comes from plant foods: Highly fibrous tubers, honey, and berries; 30 percent from birds and game animals.
Now, what do we see in the Hadza, and here’s a reference for this paper here. We see increased Treponema, which is considered an opportunistic infection in industrialized countries. It’s a Spirochaete bacterium which an cause syphilis. But, it’s proficient at breakdown of xylan and cellulose. So interestingly, this infection is actually symbiotic for the Hadza.

They also have higher levels of that Bacteroidetes and a lower level of Firmicutes with enrichment in Prevotella. This will be more important when we discuss the implications of the microbiota to obesity, because in obesity it is often said that, if you have high Bacteroidetes and low Firmicutes, that’s protective against obesity. OK. And we see some studies like this, where that conclusion is being drawn from. But I will show you evidence later that will contradicts that, and it’s not really that simple. And maybe the most shocking: They have no Bifidobacterium. Bifidobacteria is one of the most well-studied, and one if the most clinically efficacious strains that we have in Westernized societies, and studies, for example, in IBS have shown a decreased diversity and count of Bifidobacteria in patients with IBS. So, we know that in Western societies, Bifidobacteria is good. You’ve probably all heard of some kind of Bifidobacterium supplements, right? But there is none of that in the Hadza, and the Hadza have very, very healthy G.I. tracts, general; they’re in excellent health. It’s been hypothesized that meat, dairy, or livestock contact may be needed for Bifidobacterium colonization and maintenance.
So, what we see here is very interesting. We see a very healthy group of hunter-gatherers. Yet, they have none of what we would consider good – the Bifidobacterium – and they have what may be considered an infection by United States standards that’s actually playing a symbiotic role for them.
You guys with me on this so far?
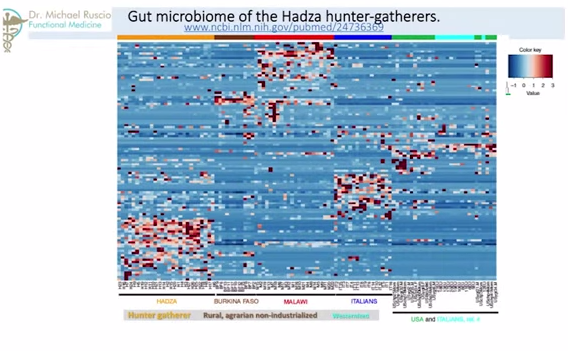
Now we will look at increases in carbohydrate digesting microbiota in populations with higher carbohydrate intake. This is what I think one of the most interesting parts of this presentation. So, going back to Hadza paper, what you are seeing – and you probably can’t see this – is a graph. The darker the color, the higher the density of that particular bacteria. So, here we are looking at Italians and people from United States. So, they have a high representation here. Here we are looking at just Italians. So, this is all Westernized, OK? Moving over here, the two other columns – here and here – this is the Burkina Faso, or Africans, against the Malawians. Two agrarian societies that are nonindustrialized – they are farmers, they are not industrialized, they are somewhat Third World. So we see a very different representation here. Now, compare that to the Hadza, who have a completely different enrichment in their bacteria.
So, what we see is from society-to-society, from Westernized-to-agrarian-to-hunter-gatherer, we see different parts of bacterial spectrum have increased or decrease densities.

So what does that mean? When we look at this next paper – I am sure some of you probably heard of the paper comparing the Africans to the Italians. When we look at the paper looking at the Africans with the Italians – and again, these are native Africans – we see here…do you see this blue outline? This is the Bacteroidetes. So, they have a lot of Bacteroidetes. And the red here are the Firmicutes, and they have a lot less compared with the Italians, which are more Westernized and have much more of this Firmicutes and much less of this Bacteroidetes. This is part of where the hypothesis comes from that high Bacteroidetes is protective against obesity. But, there may be more to it than that.
So, part of what we see here in this Bacteroidetes is this Prevotella is a major constituent here. Prevotella is needed to breakdown carbohydrate, possibly to breakdown grain. So we know the Burkina Faso have a high consumption of carbohydrate, high consumption of grain, and a high representation of this Prevotella. But, when we look at other populations that are similar, like the Russian population – that has a similar diet, high in grain, high in carbs, high in potatoes – they don’t experience this enrichment in Prevotella or this increase in Bacteroidetes. More importantly, when we look at paleo fecal samples, we do not see the same shifts of increased Bacteroidetes.
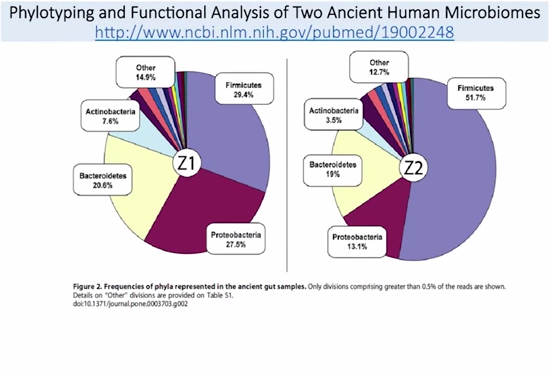
This is a fantastic paper: They actually got samples from two people who lived in the paleolithic era. What do you see here? Bacteroidetes is fairly underrepresented compared to the Africans, right?
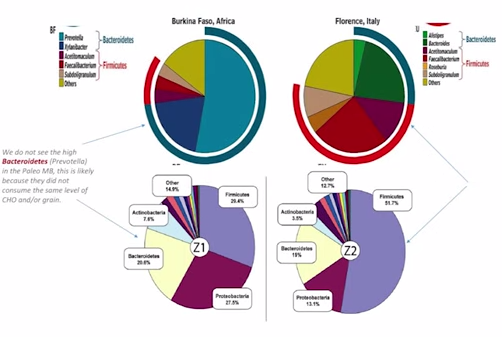
Here are the Africans. This is the Bacteroidetes here, this blue bar on the outside. So, (a) major constituent to the Africans. In the paleo fecal samples, we don’t see that same enrichment. So, what does this tell us? Well, we can draw some inferences from this. It may mean that the people in the paleolithic era did not eat the same high level of carbohydrates that we see in modern-day Italians. That’s the inference I draw from this. We also see from one sample to the others – these are two different people, Z1 and Z2, two different people – we see that the Firmicutes, which is the purple part here, shifts greatly. We don’t know why that is. What’s been shown in the Hadza, though, is that, because the women do more foraging, and the men do more hunting, there’s actually a sex difference in the representation of microbiota. The women have more of that Treponema to help break down more of the xylan and cellulose – because they are doing more foraging, snacking while they eat, and they need more of that. Men are are doing more hunting, eating more meat, so this may be a sex differentiation we are seeing here. But to zoom out: The conclusion I am trying to draw from this comparative analysis is oftentimes when we study Third World countries, they are dirty and they have more diverse colonization, which is generally good, right? But, they also don’t have as much money, so they eat cheap food – lots of carbs, and grains, and things like that. So, sometimes a conclusion that’s made is, well, they are healthy; they eat lots of carbs; we should eat lots of carbs, too. And I’m not saying that’s wrong. But I think there are some flawed assumptions being made within that paradigm, OK? Because, it may be that the people in the Third World countries derive most of their health, because they grow up and live in a dirty environment – which has continuously been shown to be very good for your immune system, and for your gut. They may be able to survive well and be healthy on whatever diet they eat, because they have this healthy microbiota, this healthy immune system, and this healthy gut.
Hopefully I didn’t lose anybody with that.

Moving on, modern dirt = bad, old dirt = good. Coming back to the comparison of US-to-Bangladesh, what we see is that the children in Bangladesh who grew up in more slum-like conditions- yes, they had much more diversity. But,they also had a high incidence of diarrheal diseases. But, the Hadza – they were also diverse, but grew up in a more hunter-gatherer society, again, had the high diversity but did not have the diarrheal diseases. What this may mean is, older dirt (is) good, (while) modern dirt (is) bad, right? And I think that makes sense. We envolved in an old-dirt scenario. We did not evolve in a modern-day dirty scenario. So, it’s a key differentiation, I think, that needs to be made, because sometimes people hear that dirt is good for you, and then they throw hygiene out the window. It may not be that simple – it’s the type of hygiene, maybe, what’s important.

So, looking at the microbiota now IBD or inflammatory bowel disease published in the journal Gut. We see here Firmicutes again. The white line is the diversity of healthy people, and the black line is the diversity of people with Crohn’s disease. You can clearly see, people with Crohn’s disease have far less diversity. The authors concluded, “We see a reduced complexity of the bacterial phylum Firmicutes in patients with Crohn’s disease. So again, reduce or decreased diversity is really a problematic issue.

Again we see from the journal of Inflammatory Bowel Disease a representation of ulcerative colitis compared to healthy people compare to Crohn’s disease, and we just see different representations in the predominant bacteria in these three different populations. But again, ulcerative colitis and Crohn’s (are) far different than healthy.

And from Nutrition Reviews, we see that Crohn’s – one of the things we see in Crohn’s, getting down to the species level – is reduced faecalibacterium prausnitzii. And in ulcerative colitis, we see decreased A. mucinuphila. Not a whole lot we can do with that just yet, but they are just observations that we are starting to piece together.
But, where we do have some good interventions for inflammatory bowel disease is with anti-inflammatory agents. And these may work very well, because it’s not a one-way street from the microbiota to the immune system. It’s a bidirectional relationship, meaning that the immune system shapes the microbiota, and the microbiota shape the immune system. What happens in inflammatory bowel disease, IBD, is peoples’ immune systems are overzealous and they start killing and pruning the healthy commensal bacteria. That’s why the anti-inflammatories seem to work well, because you are toning down that overzealous immune response.
Now, the microbiota in obesity – there is so much information that I’ve gone through; I’m just going to give you the executive level summary here. The Firmicutes/Bacteroidetes ratio, OK? This is something that you will, maybe, hear a lot about. When you go to the medical literature, you see the animal model hypothesis definitely supports an alteration of high Firmicutes/Bacteroidetes ratio correlated with obesity. When you go into the human level

studies, there are several contradictions, so that hypothesis does not hold its weight in human studies. So, we can’t really apply that to humans.
But, in healthy-versus-obese macrobiota, again, there’s no consistent difference, but there is consistently a difference. So consistently, obese are different than healthy, it’s just that the difference is not consistent, with except the one exception of decreased diversity, which seems to be a reoccurring theme here – that decreased diversity is kind of the kiss of death when it comes to your microbiota. Now, short chain fatty acids is another contentious issue regarding obesity. Again, short chain fatty acids are just carbohydrate foodstuffs that we can’t digest – the bacteria digest for us, and they secrete short chain fatty acids, which give us some energy,and which also are very reparative to the intestinal cells.
So, one of those is butyrate. And butyrate levels have been consistently shown to be elevated in the obese. It’s somewhat paradoxical because butyrate is suppose to be so healthy for the colon. Yet, people who are obese, have high levels of butyrate. The high levels of butyrate may be because people who are obese are eating lots of carbohydrates – or more food in general, and supply more substrate to produce a short chain fatty acids. But, it also may be that people who are obese have an increase ability to harvest energy from food – you’ve probably heard something along these lines before. Here’s, maybe, a support for that: The Hadza also had high short chain fatty acids, but they had high propionate.
Now, this is my hypothesis as to what’s happening here: The Hadza don’t have as much food, they live in a much more harsh environment. So, for them to be able to extract as much calories from their food as possible would be a survival advantage, right? So, they make a lot of short chain fatty acids; (they are) very good extracting all of the calories they can from food. But put them in Westernized society, where there’s plenty of food and they are extracting a ton of energy from it, they may be more prone to become obese. So, the high ability to extract short chain fatty acids from foods may be an adaptation to help you survive through famine. But, when you put that into a modern-day society, it may become problematic.
The other interesting thing about short chain fatty acids is low-carb diets will decrease short chain fatty acids, which is one of the criticisms of low carbohydrate diets in the long term, because you may do damage to your healthy bacteria, and you may do damage to your colon, because you starve all the short chain fatty acids that are needed to repair the colon. However, paradoxically, they are also very effective for weight loss.
Paradoxically even further yet still, some interventions, with things like prebiotics and fiber – that increase short chain fatty acids – have shown a modest ability to decrease weight. So the picture of short chain fatty acidsin obesity is not incredibly clear at this point. What is a little more clear is inflammation. I’m sure some of you have heard that people who are obese tend to have more inflammation in the gut – more of what’s called endotoxemia or more of these LPS, lipopolysaccharide. What may be happening in people that are obese is they don’t have a lot of diverse exposure to bacteria early in life, and when they don’t their immune system tends to be more inflammatory. So they eat, and that simulates inflammation, and then inflammation can cause insulin resistance and can cause the slowing of metabolism. So, what may happen in obesity is the obese may have poor colonization, (and) that poor colonization causes them to be more inflammatory, and that inflammation sets the stage for metabolic derangement. One of the ways we can guard against that is early, diverse exposure to bacteria, which is a segue into the next section which is microbiota and autoimmunity.

So, in the Journal of Allergy and Clinical Immunology, what they are showing here – again, this is diversity, so the higher you go, the more diverse. And this is age. Now, in children that only achieve a minor amount of diversity, they are more prone to become allergic later life. What typically happens is this: You have a window, and once you pass this window – (at) maybe about two or three years of age – you don’t really gain any more diversity. You can’t really gain much diversity after three. You hit three, the diversity you have, you are kind of locked in with. (If you) give that a few years, that can become allergies and asthma. Compared to the people who hit this higher level diversity, give that a few years and they become healthy. This has been supported by numerous studies.

Another one of which is here. This is looking at prenatal farm exposure and its relationship to allergy and immunity. What you are seeing here is a number of different farm animal species the mother had contact with during pregnancy okay. So, the farther we go to the right, the more animals – 0-to-6, 0-to-6, 0-to-6. What you are seeing here are receptors for the immune system – TL receptor 2, 4, and CD14 cells. The more of these you have, the more trained your immune system is to be able to distinguish self from nonself, which is one of the fundamental tenets of autoimmunity. When your immune system doesn’t have good aim – is that a thyroid or is that a bacteria? And it just starts shooting whatever, starts shooting your thyroid tissue – now you have Hashimoto’s. The more of these TL receptors you have, the better the immune system is at distinguishing self to nonself. What you see here is the more animals mom is exposed to, the more of these receptors are expressed. What ends up happening is these become protective factors. So, farm milk consumption, regular contact with animals, frequent stable or barn visits for haying, and especially maternal stable work during pregnancy causes a very profound effects on allergies and asthma. The earlier and the more diverse exposure, the better. So, again, what this is saying is the more dirty mom got, the healthier the child was in terms of allergies and asthma. Because we had increased expression of these receptor cells that help the immune system say, “You’re a piece of good tissue – I’m not going to destroy you.’ “You are a bad guy – I’m going to attack you,’ which is the fundamental confusion that happens in autoimmunity. Are you guys with me on that? OK.
Now, backing up for one second her, that has implications potentially for obesity, because if these receptors are working well, and the immune system is working well, you become less inflamed. If inflammation can feed obesity, then we know that wherever we can do to stop inflammation will potentially help prevent obesity. So, early exposure like we are showing here, can help with allergies and asthma as verified in the peer-reviewed literature. And there is some evidence showing that may also help obesity.

So, this then leads to the question: Parasites – friend or foe? I spend a lot of time treating patients for infections, yet a lot of this information would make you think you want to have as many infections and bacteria as possible, because it’s very good for you. I’m sure many of you have seen this before. This is published in the New England Journal of Medicine showing as infectious diseases are going down allergic and autoimmune diseases are going up. This is what we see in many Westernized countries – as we have less exposure to dirt and germs, we have less infectious disease. But because with that you’re becoming so much more sterile, that sort of cripples your immune system, and now you are more prone to have allergic and and autoimmune conditions. You sacrifice one for the other.
Certainly, see other data points that support this. To quote, “These results suggest a protective benefit of H. pylori infection against development of inflammatory bowel disease.” So, the more H. pylori infections there were in this study, the less inflammatory bowel disease, because H. pylori may be a proxy for a dirty environment.

But, here’s where these get a little more mucky. Another study – 10 patients who had Hashimoto’s, which is thyroid, and the H. pylori infection were studied. Half were then treated for H. pylori, the other were not. Those treated for a H. pylori saw a marked and significant decrease in their thyroid autoimmunity. So, how do we account is? How can H. pylori be protective against some autoimmune conditions but trigger potentially other autoimmune conditions?

Well, I think has to do with timing, one, and context, two. So, timing – if these inoculations occur before three years of age, and before the gut stabilization window closes at about three years of age, they tend to be protective, OK? So beneficial before three years of age, but later in life, they can potentially be detrimental. This, by the way, has been reinforced with Epstein-Barr virus colonization and multiple sclerosis. Early colonization with Epstein-Barr virus protects against multiple sclerosis; late colonization is a risk factor for multiple sclerosis. In context, the person’s immunogenetics, meaning do they have more of a tendency to have a very strong pro-inflammatory immune response and the diversity of the pre-existing colonization; the more diverse your colonization is, it’s kind of like one more pathogen in the mix, probably won’t be a big deal if you have a lot of players. What can happen is – you probably can’t see this but – if there’s a lot of bacteria already living in the colony, one more is somewhat policed from running amok, because you have all these other guys that are trying to push them out, if that makes sense. So, in summery, if you have late colonization or infection, plus poor microbial diversity, plus the appropriate genetics, you have sickness. These microbes that maybe good for some, may become become harmful for you. That’s the real thing I think we are juggling in Westernized societies: The Treponema infection in the Hadza, benefitial. If someone here acquired that tomorrow, it’s probably going to be pathogenic for you. So we have to be able to separate out what population were dealing with to know how to treat the person appropriately.
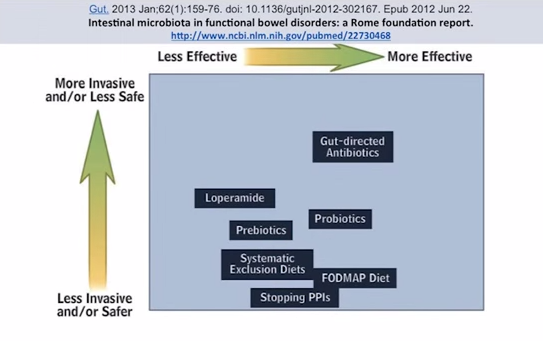
So, manipulation of the microbiota. My clincial observations: If diet doesn’t fix the problem – and I should say diet, lifestyle, maybe some probiotics, maybe some additional enzymes – if those basics don’t fix a problem, there is likely a gut infection overgrowth or imbalance at the root of the problem. This is from the journal Gut, in regards to functional bowel disorders. Things that are over to the right here are more effective, and higher up here are more invasive. We want to be…this is the sweet spot. This is more effective and least invasive. And what do we see? Fodmap diet, works pretty well. Probiotics, and also gut-directed antibiotics, but they are a little bit more toward the more invasive end. So, what I would submit is a substitute that would, maybe, fall into this area would be antimicrobial herbs – herbs that can kill things but don’t have quite the same detrimental effect that pharmaceutical antibiotics may have.

Diet is powerful, and it can influence the macrobiota, and it can shift the microbiota. The challenge is, we don’t know enough to be able to say, ‘You have this condition. We’re going to give you this diet to manipulate the microbiota in this way, to create this kind of outcome.” We don’t have that level of data yet. But what we do have are observational studies, where people go on this diet, with this condition, (and) they get better. So some of what we see with the diets – preaching to the choir here, I think – paleo seems to be pretty much good for everything – It’s a very good starting point. It’s good for general health, weight loss, autoimmunity, and digestive conditions. The autoimmune paleo would be a more strict application of paleo, which is usually what I will start a patient on in the clinic. We will put them on the autoimmune paleo diet, (and) we’ll see how they do 30 days later. For some people, this will completely, 100 percent address their chief complaint in 30 days or so. I’m sure you guys have either had experiences yourself or know people who have. Some specialty diets would be the low Fodmap diet, or the SCD diet, which starves bacteria and is best for SIBO and IBS. Ironically, we see so much about how diversity of bacteria is healthy, but our clinical intervention tend to be interventions that are geared at killing bacteria. That may have to do with the fact that we have evolved, most of us anyway, in a very sterile environment, so we may not be able to tolerate this highly diverse bacterial colonization. We may not have a lot of good bacteria to crowd out bad bacteria, to have his reoccurring problem of bad bacteria continually popping up because we don’t have enough good bacteria to crowd them out.
SCD and GAPs diet also starves bad bacteria, and the GAPs provides a lot of good bacteria, helpful for SIBO and IBS. SCD may be a little bit better for inflammatory bowel disease as some studies have shown. And then, fasting is another intervention that can be useful for people. Remember, we evolved in famine and feeding cycles, so fasting is probably somewhat of a normal part of our DNA, if you will. And, one of the things I use clinically is, if a patient comes in with a lot of gastrointestinal complaints, we may put them a three-or-four-day liquid diet because that’s much easier for their system to be able to break down foods like that. The analogy I use with my patients often is, if you sprained your ankle, but you had to run a mile every day, how long will it take your ankle to heal, right? So, if we can get you off that ankle, if we can give your gut a break from having to digest these hard foodstuffs for a few days, that can be really powerful in allowing the gut to repair itself. So, how about weight loss? Well, starting with low-carb autoimmune paleo diet is a really good starting point. Then, slowly add cabs back into tolerable levels because, remember, if you’re very low-carb for too long – if you are consistently under, maybe, 100 grams, that can cause a loss of healthy bacterial populations, and it can cause a decrease in some of the protective short chain fatty acids. So, you don’t want be any more low-carb than you necessarily have to be.

Can I manipulate the microbiota for weight loss? Well, let’s look at that. The microbiotial manipulations in obesity. We already know the antibiotics used early in life is a risk factor for obesity. And diverse early exposure may be protective. Why is that? Because, if you don’t have diverse early colonization, you don’t make that immune system training, and that makes your immune system more prone to inflammation, and the inflammation can cause things like insulin resistance and impaired metabolism.
Low carbohydrate diets show some promising results. Fourteen pounds lost in a very low-carb diet study; a low-calorie and low-carb study showed 16 pounds lost, and the low calorie with low fat showed 13 pounds loss. Now, important for me to mention this here: Negative changes to the microbiota from very low-carb diets can be offset by supplementation with fiber, prebiotics, and resistant starch. They have done studies where they put obese patients on low-carbohydrate diets, but they’ve given them various prebiotics or resistant starch, and they’ve seen the bacterial populations that normally die flourish. So, there are some great implications for if you’re someone that really can’t tolerate carbohydrate, and you have to stay on a long term low-carbohydrate diet, prebiotics and resistant starch may help prevent the loss of short chain fatty acids and other helpful bacteria.

How about fiber? The worst studies regarding fiber show no weight loss; the best results have been with fiber’s glucomannan. There were 8.3-more pounds lost than the control group. So, two groups, bith on a low-calorie diet – one went on a low-calorie diet with glucomannan fiber. That group lost 8.3 pounds more than the control group. So, not bad. Other reviews have shown fiber’s average weight loss – again, reviews pool many, many studies – weight loss would be about 4.2 pounds. So, the average loss, maybe about 4.2 (pounds), glucomannan mayget you to 8.3 (pounds), and viscous fibers may be better than non-viscous. A viscous fiber is one that when you mix it with water, (it) forms this kind of thick gel. A non-viscous one is very, very liquid still.
Prebiotics – The best studies using prebiotics only show about 2.5 pounds lost. So, it doesn’t seem prebiotics can have a massive effect on weight loss.
Probiotics – The best results for weight loss here was with Lactobacillus rhamonsus, at about 3.7 pounds. Lactobacillus gasseri was about 2.2 pounds. So, not huge amounts of weight loss that we can achieve with probiotics/prebiotics. But fiber shows some benefit, and the one that shows the most benefit is really low-carbohydrate diet, in this case.
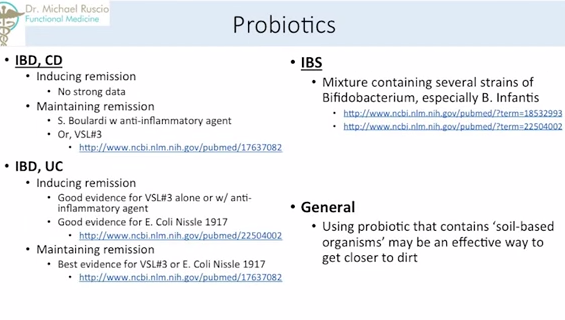
Probiotics for Crohn’s disease – The best data we have is in maintaining remission, with S. boulardi and an anti-inflammatory agent or with a probiotic known as VLS#3. VLS#3 is a blend of different lactobacillus and bifidobacterium species.
For ulcerative colitis, there is good evidence for VLS#3 along, or with an anti-inflammatory agent. And there’s very good evidence for E. Coli Nissle 1917. There’s a lot of great research on E. Coli 1917, also known as multiforme. Unfortunately, it is no longer available in the United States, but there are some very impressive studies on with this probiotic. You can still get it; you just have to go through another country.
And for maintaining remission in ulcerative colitis, the best evidences is for VLS#3 or E. Coli Nissle 1917. For IBS, (it’s) a little different. Bifidobacterium, especially bifidobacterium Infantis, seems to have the best results. In general, using probiotics that contain soil-based organisms may be an effective way to get closer to dirt. So, one of the the things that we don’t have is enough exposure to dirt. So, one of the things you can do is roll around in the dirt, I guess. Or, you could take a probiotic that contains some of these soil-based organisms. There’s not a ton of clinical trials with these just yet. But, I suspect as we see more and more with these, we will see better and better things. What I do in the clinic is I usually always use a soil-based probiotic mixed with one of these, depending on the condition, with or without prebiotics, depending on the condition also. So, kind of my base there.

The microbiota in autoimmunity – In the functional medicine community, the role of infections in stimulating autoimmunity is an area of great interest at the moment. H. pylori, Epstein-Barr virus, yersinia and terra colitis and other infections have shown very strong correlations to autoimmunity…very strong. What we are lacking is a lot of strong data showing that, if you treat that infection, autoimmunity then goes away. There have been a few studies done – we discussed the one about treatment with H. pylori infection in patients with Hashimoto’s thyroid autoimmunity, and we saw a benefit there. Some of these studies are starting to pop out; there are just kind of slow to be done. But, one of the things I do see routinely is that, when we treat infections, more often than not we will see improvement in autoimmune conditions. It makes sense if an infection is going to chronically stimulate your immune system, and if autoimmunity is just an issue of overzealous immune function, clearing an infection, allowing the immune system to go into relaxation mode, makes sense that it would help an autoimmune condition.
The best preventative measures are early and diverse exposure, like we showed with the mothers that had diverse exposure to farm animals while they were pregnant. That’s the best. But, again, if you are 30 years old, and you just came down with an autoimmune condition, you can’t really do a whole lot about your upbringing,

And, a quick word about antimicrobials, which are nature’s antibiotic. When pretty much nothing else works, again, look to an infectious issue in the gut. Common infections – SIBO or small intestine bacterial overgrowth, fungus or candida, worms, amigos, bacteria, and protozoa. These are things that I find fairly common in patients that have chronic health issues that just won’t respond. Natural antimicrobial agents may be as effective as prescriptions in killing what you don’t want, but also better and not causing imbalances, which is referenced by this paper here. Natural agents also have additional benefit – Burberine helps lower blood sugar; Artemisia or wormwood is also a very powerful anti-inflammatory. The nice thing about herbal medicines, in the application of getting rid of bad bacteria, is they may not kill your good bacteria, and they give you additional health benefits, like anti-inflammatory or increasing your insulin sensitivity.
And remember that the immune system and the microbiota have this back and forth. And, if your immune system is overzealous, it can damage your microbiotoa. In this meta-analysis, they compared herbals to things like mesalazine. They concluded the overall efficacy and tolerability of herbal medicine in IBD is comparable to 5-ASA drugs. Again, things like mesalazine. So, some of what was used was Artemisia, boswellia, andrographis, and aloe as agents that can help dampen this overzealous immune response that some of us have in the United States due to non-diverse exposure during our formative years.

So in summary, the microbiota has evolved with humans to allow survival. The microbiota provides important functions in digestion, immunity, metabolism, and detoxification. We should be careful when interpreting microbiotal patterns seen in less-developed countries. Increased diversity is seen as clearly good. Adaptation to higher carb intake is unclear. Early and diverse exposure are key to a healthy microbiota. Getting dirty with old dirt is good for you. The relationship between obesity and the microbiota is unclear, other than a decreased diversity is seen in the obese. And manipulation of the microbiota shows promise for modest weight and metabolic improvement. And, more significant potential for anti-inflammatories and autoimmune conditions using anti-inflammatory agents, antimicrobials – and, I should also add probiotics. And finally, diet is a powerful factor that can shift the microbiota.
So, it was a lot of information. Hopefully you guys learned a few things from it.
And, thank you very much for your time.
Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!