Practitioner Research Review – November 2018
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
In Today’s Issue
Research
- Re-Challenge Studies in Non-Celiac Gluten Sensitivity: A Systematic Review and Meta-Analysis
- To Treat or Not To Treat H. pylori – Dr. Ruscio’s Review of H. pylori Treatment Consensus Data
- Clinical experience with the use of anti-CdtB and anti-vinculin antibodies in patients with diarrhea in Mexico
- Rapid-Fire Research – Ultra-concise summaries of noteworthy studies
- Update on dietary therapy for eosinophilic esophagitis in children and adults
- Prucalopride succinate for the treatment of constipation: An update
- Parity and 11-Year Serum Thyrotropin and Thyroid Autoantibody Change: A Longitudinal Population-Based Study
- Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials
- Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however, the research studies are a collection of the most clinically meaningful research that has been published recently.
Re-Challenge Studies in Non-Celiac Gluten Sensitivity: A Systematic Review and Meta-Analysis
https://www.ncbi.nlm.nih.gov/pubmed/28928668
Study Purpose
- To see how common non-celiac gluten sensitivity (NCGS) is upon reintroduction of gluten.
- Note: This provides a much more accurate measure as compared to those who merely proclaim a problem with gluten but never perform a reintroduction test.
Intervention:
- Systematic review with meta-analysis of 11 studies
Main Results:
- Roughly 30% of subjects, with a prior diagnosis of NCGS, relapse after reintroduction
- The overall pooled percentage of patients with a diagnosis of NCGS relapsing after a gluten challenge was 30%, with a wide range across the studies varying between 7 and 77%.
- However, when accounting for placebo effect it appears roughly the same percentage of subjects reported a reaction to gluten as to placebo – meaning the placebo is highly influential.
- When comparing the relapse between patients receiving gluten with respect to those receiving placebo, RDBPC (randomized, double-blinded, placebo-controlled) studies with or without a cross-over design found a similar percentage of relapse after either a gluten or a placebo challenge (RDBPC with cross-over: 34 vs. 32%, respectively; p = 0.7. RDBPC without cross-over: 38 vs. 28%, respectively
- Importantly, there was a significantly higher risk (16%) of relapse after reintroducing gluten than compared to placebo, but only if the Salerno criteria for reintroducing was followed. Note: There were essentially 2 forms of reintroduction measured 1) a standard double-blinded placebo-control reintroduction or 2) the stricter Salerno which is also double-blinded and placebo-controlled but even more rigorous (see below).
- The overall pooled percentage of patients with a diagnosis of NCGS relapsing after a gluten challenge performed according to the recent Salerno criteria was significantly higher as compared to the percentage of patients relapsing after placebo (40 vs. 24%; p = 0.003), with a significant RR of relapse after gluten challenge as compared to placebo (RR = 2.8; 95% CI = 1.5–5.5; p = 0.002).
- What are the Salerno criteria? The criteria are published and used to diagnose NCGS.
- The standardization of the diagnostic protocol has been recently published by a group of experts on gluten-related disorders, and is called “Salerno criteria”.
- Salerno NCGS diagnosis protocol:
- First the patient should exhibit at least a 30% decrease in gastrointestinal and/or extra-intestinal symptoms after a 6-week GFD.
- Then, a double-blind placebo-controlled (DBPC) re-challenge with crossover should be performed, with 1 week of duration for each challenge and the wash-out period in between.
- The recommended daily doses for gluten are 8 g, whereas the placebo should be gluten-free.
- In recap, those who started the study as self-reported NCGS were only found to have a problem with gluten if the Salerno criteria for reintroduction were followed. Unless these were followed, the same amount of subjects reported a ‘reaction’ to gluten as they did to placebo. So, the Salerno criteria guard against the placebo effect. This is very important.
- How were patients at study entry labeled as NCGS? Essentially those who self-reported they were and did not have celiac or a positive IgE skin test.
- NCGS was defined as self-reported gluten intolerance resulting in gastrointestinal and/or extra-intestinal symptoms, which remitted upon gluten withdrawal, with a documented exclusion of WA (IgE skin testing) and CD (seronegativity and absence of villous atrophy;
- Did FODMAPs skew the results? A FODMAP containing reintroduction substance was used in 2 of the 11 studies.
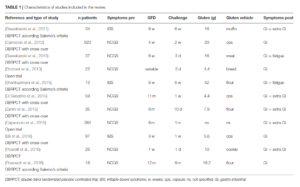
- Here is the data table summarizing the 11 studies:
Authors Conclusion:
- “The prevalence of NCGS after gluten re-challenge is low, and the percentage of relapse after a gluten or a placebo challenge is similar. However, a higher number of patients will be correctly classified with NCGS if applying the recent Salerno criteria.”
Interesting Notes:
- “For the experienced clinician, extra-intestinal symptoms serve as the best indicator of the disease, and are increasingly recognized as hallmarks of NCGS”
- “The amount of gluten administered was ≥ 8 g/die in all studies except three (Di Sabatino et al., 2015; Zanini et al., 2015; Elli et al., 2016); the duration of challenge was ≥ 1 week in all, except one (Picarelli et al., 2016). Symptoms evaluated after challenge were prevalently IBS-like.”
Clinical Takeaways:
- NCGS is a legitimate and verifiable diagnosis, however the details matter
- The placebo effect is highly influential in leading someone to believe they have NCGS, thus leading to a roughly 30% of subjects reporting a reaction when not controlled for.
- When placebo is controlled for, there appears to be no difference between placebo and gluten reintroduction, unless the Salerno criteria is followed, then the change of reaction is 16%.
Dr. Ruscio Comments
It likely is not practical to have your patients perform a placebo-controlled reintroduction according to the Salerno protocol. However, the takeaway here is twofold:
- Yes, NCGS is a valid condition
- It can be over reported so be cautious with your interpretation of a patients experience and how fervently you encourage gluten avoidance.
To Treat or Not To Treat H. pylori – Dr. Ruscio’s Review of H. pylori Treatment Consensus Data
Links Provided Below
Study purpose
- My short review of the data regarding deciding to treat or not treat H. pylori.
Intervention:
- The original article prompting my attention was Treating Helicobacter pylori effectively while minimizing misuse of antibiotics, which concluded all H. pylori infections should be treated.
- Experts now recommend that all Helicobacter pylori infections be eradicated unless there are compelling reasons not to. As with other infectious diseases, effective therapy should be based on susceptibility.
- This conclusion was based on 4 other review papers (1, 2, 3, 4). Here are select highlights from each paper below.
The Toronto H. pylori Consensus in Context
- If using an antibiotic, use a culture-guide susceptibility test to determine what the best antibiotic will be. A recent meta-analysis showed better outcomes when doing so. This is an example of when additional testing can be worthwhile.
- However, this paper continues that this testing is not always readily available and this recommendation may be challenging to implement in the clinical setting.
- They continue,
- The most common causes of treatment failure are antibiotic resistance and lack of patient adherence to the regimen
Kyoto global consensus report on Helicobacter pylori gastritis
- While H. pylori may cause ulcerations, gastritis, and duodenitis – I don’t feel enough attention has been given to its potential role in dyspepsia (indigestion). This paper proposes formally considering this association. Meaning – you are justified in an H. pylori workup in patients with indigestion.
- A new category of H. pylori-associated dyspepsia together with a diagnostic algorithm was proposed.
- This paper also provides a good reminder that gastritis and duodenitis can be caused by viruses and fungi as well as other bacteria. Especially regarding infectious gastritis, remember it’s not only caused by H. pylori. Autoimmunity, NSAIDs, stress, and alcohol are also considerations.
The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults
- Due to the decreasing effectiveness of antibiotics, longer and more aggressive H. pylori treatment is now being recommended.
- Because of increasing failure of therapy, the consensus group strongly recommends that all H. pylori eradication regimens now be given for 14 days. Recommended first-line strategies include concomitant non-bismuth quadruple therapy (proton pump inhibitor [PPI] + amoxicillin + metronidazole + clarithromycin [PAMC]) …
- However, they don’t mention the important finding that probiotics have been shown to enhance H. pylori antibiotic treatment (1). Nor is there mention of NAC co-administration, although understandable as the data here are sparse.
- Even though the data for natural therapies is not perfect, a course of natural therapies is justifiable in my opinion.
Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report
- **Most insightful of these papers** There is a lot here…
Testing
- Again, testing for H. pylori in dyspepsia is being recognized
- A test-and-treat strategy is appropriate for uninvestigated dyspepsia in populations where the H. pylori prevalence is high (≥20%). This approach is subject to local cost-benefit considerations and is not applicable to patients with alarm symptoms, or older patients (age to be determined locally according to cancer risk)
- Preferred tests are urea breath test (UBT) and the stool antigen test. They are equivalently accurate.
- The main non-invasive tests that can be used for the test-and-treat strategy are the UBT and monoclonal stool antigen tests. Certain validated serological tests can also be used.
- The diagnostic accuracy of the stool antigen test (SAT) is equivalent to the UBT if a validated laboratory-based monoclonal test is used.
- Blood antibody testing should be limited to IgG testing
- The serological tests are not all equivalent. Only validated IgG serology tests should be used owing to variability in the accuracy of different commercial tests.
- For patients on PPIs, they should be stopped 2 weeks prior to stool, biopsy or HP breath testing. If this is not possible, IgG testing can be used.
- In patients treated with PPIs: (1) if possible, PPI should be stopped for 2 weeks before testing by culture, histology, rapid urease test, UBT or stool test.
- If it is not possible, validated IgG serology can be performed.
- HP breath test or stool antigen test can be used to confirm eradication, blood testing should not be used.
- The UBT or a laboratory-based validated monoclonal stool test are both recommended as non-invasive tests for determining the success of eradication treatment. There is no role for serology.
- Retesting should occur 4 weeks or longer post-treatment
- The time for testing the success of H. pylori eradication after the end of treatment should be at least 4 weeks.
- Pepsinogen testing can identify if gastric atrophy is present.
- Validated serological tests for H. pylori and markers of atrophy (ie, pepsinogens) are the best available non-invasive tests to identify subjects at high risk of gastric cancer.
Other
- Probiotics and prebiotics are a consideration
- Certain probiotics and prebiotics show promising results as an adjuvant treatment in reducing side effects.
- Certain probiotics and prebiotics show promising results as an adjuvant treatment in reducing side effects.
- Treating H. pylori for dyspepsia is relatively effective when compared to other treatments (but absolute numbers not impressive, in my opinion).
- H. pylori eradication produces long-term relief of dyspepsia in one of 12 patients with H. pylori and functional dyspepsia; this is better than any other treatment.
- Eradication of H. pylori can increase or decrease acid, depending on the region of the stomach affected
- H. pylori can increase or decrease acid secretion depending on the intragastric distribution of inflammation.
- No strong association to gastro-oesophageal reflux disease GORD aka GERD
- On average, H. pylori status has no effect on symptom severity, symptom recurrence and treatment efficacy in GORD. H. pylori eradication does not exacerbate pre-existing GORD or affect treatment efficacy.
- Epidemiological data counterintuitive, perhaps because H. pylori may be a proxy for a less hygienic environment with is also immuno-protective.
- Epidemiological studies show a negative association between the prevalence of H. pylori and the severity of GORD and incidence of esophageal adenocarcinoma.
- H. pylori eradication protects against ulcers
- H. pylori infection is associated with an increased risk of uncomplicated and complicated gastroduodenal ulcers in NSAID and low-dose aspirin (acetosalicylic acid (ASA)) users.
- Eradication reduces the risk of complicated and uncomplicated gastroduodenal ulcers associated with either NSAID or low-dose ASA use.
- **Long-term use of PPIs in HP-positive patients accelerates atrophic gastritis** However, eradication of HP prevents progression to atrophic gastritis in long-term PPI users
- Long-term treatment with PPIs in H. pylori-positive patients is associated with the development of corpus-predominant gastritis. This accelerates the process of loss of specialized glands, leading to atrophic gastritis.
- Eradication of H. pylori in patients receiving long-term PPIs heals gastritis and prevents the progression to atrophic gastritis. However, there is no evidence that this reduces the risk of gastric cancer.
- H. pylori should be considered in those with otherwise unexplained iron or B12 anemia.
- There is evidence linking H. pylori to the etiology of otherwise unexplained iron-deficiency anaemia, idiopathic thrombocytopenic purpura (ITP) and vitamin B12 deficiency. In these disorders, H. pylori should be sought and eradicated.
- There is no evidence that HP plays a protective role against asthma, atopy or obesity
- Statement 14: The evidence available shows no definite causative protective effect of H. pylori against the following disorders nor that its eradication causes or worsens them. However, further research is needed.
1. Asthma and atopy
2. Obesity and related illnesses
- Statement 14: The evidence available shows no definite causative protective effect of H. pylori against the following disorders nor that its eradication causes or worsens them. However, further research is needed.
- H. pylori treated patients have improved bio-availability of thyroid hormone medication
- In H. pylori-positive patients eradication treatment improves the bioavailability of thyroxine
- The authors also expand upon best practices for antibiotic and drug treatment, which for brevities sake I will not list here, but details are available in full paper.
- Whether or not to use PPIs after eradication of H. pylori depends on context. What if the patient does not want to be on PPIs? Here is my opinion – If the conventional medication recommendation is to use PPIs, this means the patient is at higher risk if not using them. So, if the patient elects to use natural treatment instead they should be sure t & encouraged to follow up with their gastroenterologist to monitor the situation. If, after a trial period, natural medicines are not leading to improvement, then PPIs are a consideration.
- In uncomplicated DU, prolonging acid inhibition with PPI is not recommended after Helicobacter pylori treatment
- In GU and complicated DU, prolonging PPI
- is recommended
- H. pylori eradication treatment should be started at reintroduction of oral feeding in cases of bleeding ulcer
- Treating H. pylori reduces gastric cancer risk.
- H. pylori infection is the most consistent risk factor for gastric cancer. Its elimination is, therefore, the most promising strategy to reduce the incidence of gastric cancer
- The virulence factors & host genetics impact gastric cancer risk but data here not clear enough to generate a recommendation.
- The risk for gastric cancer development is influenced by bacterial virulence factors, but no specific bacterial virulence markers can be recommended for clinical practice.
- The risk of gastric cancer is influenced by host genetic factors but in clinical practice, no specific marker can be recommended for genetic testing at present
- H. pylori is more important than environmental factors in the development of gastric cancer.
- The influence of environmental factors is subordinate to the effect of H. pylori infection.
- H. pylori eradication removes and may reverse the irritation to the stomach, aka gastritis, which can cause hypochlorhydria
- H. pylori eradication abolishes the inflammatory response and slows or may arrest the progression of atrophy. In some cases, it may reverse atrophy.
- H. pylori eradication to prevent gastric cancer should be considered in the following:
- first-degree relatives of family members with a diagnosis of gastric cancer; patients with previous gastric neoplasia already treated by endoscopic or subtotal gastric resection;
- patients with a risk of gastritis: severe pan-gastritis, corpus-predominant gastritis, severe atrophy;
- patients with chronic gastric acid inhibition for more than 1 year;
- patients with strong environmental risk factors for gastric cancer (heavy smoking, high exposure to dust, coal, quartz, cement and/or work in quarries);
- H. pylori-positive patients with a fear of gastric cancer.
Clinical experience with the use of anti-CdtB and anti-vinculin antibodies in patients with diarrhea in Mexico.
https://www.ncbi.nlm.nih.gov/pubmed/27681080
Study Purpose
- Assess utility of testing for CdtB and vinculin antibodies
Intervention:
- Retrospective chart review of 30 patients
Main Results:
- Roughly 50% of those with IBS were positive for the antibodies
- Anti-CdtB/anti-vinculin antibodies were present in 8 patients (47.1%) with ‘‘pure’’ IBS. However, if we add the positivity among those with IBS-D Overlap, 55% of all patients with a diarrhea-related IBS diagnosis were positive.
- This test can help to prevent further testing to evaluate other cause of diarrhea like celiac and inflammatory bowel disease. Note: this is different than how many in the SIBO community are using it – to substantiate a need for prokinetic therapy.
- The inclusion of this blood panel in the diagnostic process of patients with IBS according to Rome III has the potential for significant cost savings because unnecessary testing to rule out organic causes of chronic diarrhea would be avoided.
Limitations:
- This was a very small study with no control group.
Authors Conclusion:
- “Our findings support the use of this test as a first-line diagnostic tool to confirm the presence of IBS-D/IBS-M according to the Rome III criteria.”
Interesting Notes:
- I’ve often said one reason to be cautious with heavy reliance on testing is that testing only provides part of the picture. Testing for autoimmunity against the GI motility apparatus is another example of this. This same paper cites a study showing those with IBS may have an elevation of 3 different types of antibodies in a paper titled “Anti-enteric neuronal antibodies and the irritable bowel syndrome” [1]
- The 3 antigens were: (1) a nondescript ribonucleoprotein (RNP-complex); (2) small nuclear ribonuclear polypeptide A; and (3) Ro-5,200 kDa.
- This is why it’s important that clinicians do not act prematurely on research/academic findings until we know how those findings translate into clinical practice and what interventions can/should be taken.
Clinical Takeaways:
- Vinculin and CdtB antibodies can be used to identify IBS-D and prevent further investigations into the causes of diarrhea.
- Caution should be exercised with generating other prognoses or treatment recommendations based upon these antibody results.
Dr. Ruscio Comments
Antibody testing for ‘the underlying cause of SIBO’ is tempting but there is still much we don’t know in terms of what the results mean and what clinical action should be taken. I would recommend only using this test, as the research recommends, as a screening tool for IBS. Other than that I would use established treatment options for IBS and SIBO, guided via history, context, response to treatment and any other validated test results.
Rapid-Fire Research – Ultra-Concise Summaries of Noteworthy Studies
Update on dietary therapy for eosinophilic esophagitis in children and adults
https://www.ncbi.nlm.nih.gov/pubmed/27998193
- More evidence showing there is no advantage to food-allergy testing, and that treatment with elimination diets are superior.
- None of the currently available food allergy tests adequately predict food triggers for EoE, especially in adults.
- Elemental diet (exclusive feeding with amino acid based formulas) and empiric six-food elimination diet, withdrawing cow´s milk, wheat, egg, soy, nuts and fish/seafood for 6 weeks, have consistently shown the best cure rates.
Prucalopride succinate for the treatment of constipation: An update
https://www.ncbi.nlm.nih.gov/pubmed/26647167
- Prucalopride, aka Resalor, is safe and effective in treating constipation
- Due to its few side effects, the lack of cardiovascular effects and interactions with other drugs, prucalopride may be safely used in elderly people as well.
Parity and 11-Year Serum Thyrotropin and Thyroid Autoantibody Change: A Longitudinal Population-Based Study
https://www.ncbi.nlm.nih.gov/pubmed/26711373
- HRT may protect against AIT, although the association was not significant. Having balanced female hormones may protect against autoimmunity.
- An inverse association was found between the number of years on HRT and the risk (odds ratio) of increased TPOAb status during follow-up (0.735 [confidence interval 0.558-0.968], p = 0.03). However, this association was not statistically significant when applying the Bonferroni adjusted significance level.
Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials
http://www.ncbi.nlm.nih.gov/pubmed/26340330
- Systematic review with meta-analysis, 15 studies with 788 subjects
- “Our findings suggest that probiotic supplementation use is effective in lowering the lipid level and coexisting factors associated with cardiovascular disease.”
- Markers effected:
- total cholesterol, low-density lipoprotein (LDL), body mass index (BMI), waist circumference, and inflammatory markers.
Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals
http://www.ncbi.nlm.nih.gov/pubmed/26473340
- Systematic review with meta-analysis, 33 randomized clinical trials
“In conclusion, this meta-analysis showed that probiotic supplementation could be useful in the primary prevention of hypercholesterolemia and may lead to reductions in risk factors for cardiovascular disease.”
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next practitioner question of the month.

Like what your reading?
Please share this with a colleague and help us improve functional medicine
Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!