Practitioner Research Review – July 2018
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
In Today’s Issue
Research
- The prevalence, incidence and natural course of positive antithyroperoxidase antibodies in a population-based study: Tehran Thyroid Study
- A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation.
- Effect of free triiodothyronine concentration on the quality of life of patients treated with levothyroxine
- Rapid-Fire Research – Ultra-concise summaries of noteworthy studies
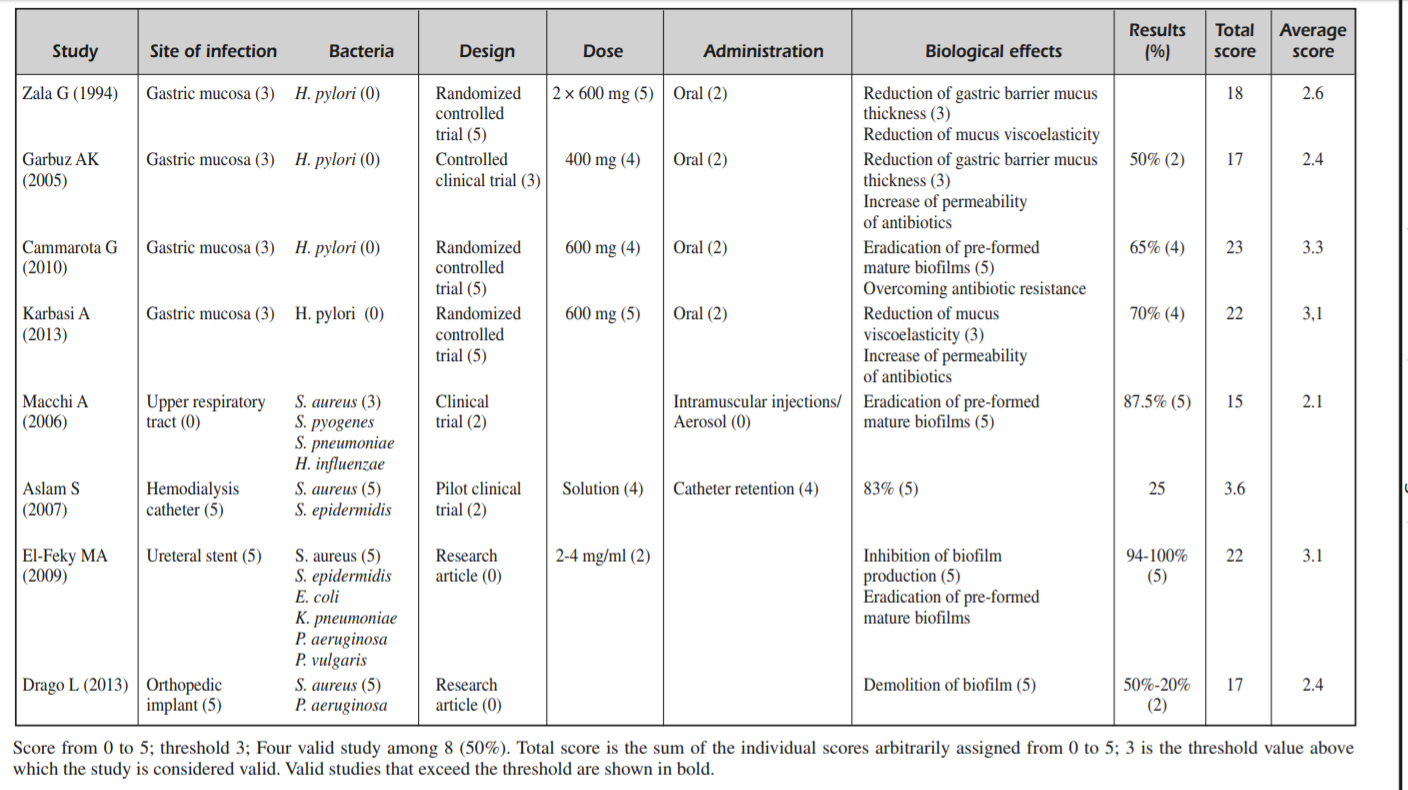
- N-acetylcysteine as powerful molecule to destroy bacterial biofilms. A systematic review.
- Female hormones influence the vaginal microbiota
- Functional 13C-urea and glucose hydrogen/methane breath tests reveal significant association of small intestinal bacterial overgrowth in individuals with active Helicobacter pylori infection.
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however the research studies are a collection of the most clinically meaningful research that has been published recently.
The Prevalence, Incidence and Natural Course of Positive Antithyroperoxidase Antibodies in a Population-Based Study: Tehran Thyroid Study
https://www.ncbi.nlm.nih.gov/pubmed/28052092
Study Purpose:
- Analyze the prevalence and incidence of thyroid autoimmunity and natural course of TPOAb in a population-based study
- This is one of the few studies providing this data
- our study is one of the few long term follow up studies which provides incidence data for TPOAb status over a median 9.1 yr follow-up in different age and sex groups, which also allowed us to determine the thyroid function of a population based on their TPOAb status.
Intervention:
- This prospective study was conducted within the framework of the Tehran Thyroid Study (TTS) on 5783 (2376 men and 3407 women) individuals aged >20 years who had thyroid function tests at baseline and were followed up for median 9.1 year with TPOAb measurements at approximately every 3 years
Main Results:
- The mean age of total population at baseline was 40.
- At baseline, of the 5783 participants, 742 (12.8%) were TPOAb positive, with higher prevalence among women than in men (16.0 vs. 8.5%,).
- The total incidence rate of TPOAb positivity in the total population (5020) was 7.1 per 1000 person-years of follow-up
- TPOAb positivity tended to increase slightly with serum TSH levels
- TSH levels tended to be highest at the point in time when one becomes positive for thyroid autoimmunity. Remember they were measuring in 3-year intervals.
- Our findings demonstrate that individuals, who became TPOAb positive in each phase, had significant elevation of TSH levels at the phase of seroconversion, compared to baseline values.
Additional Results:
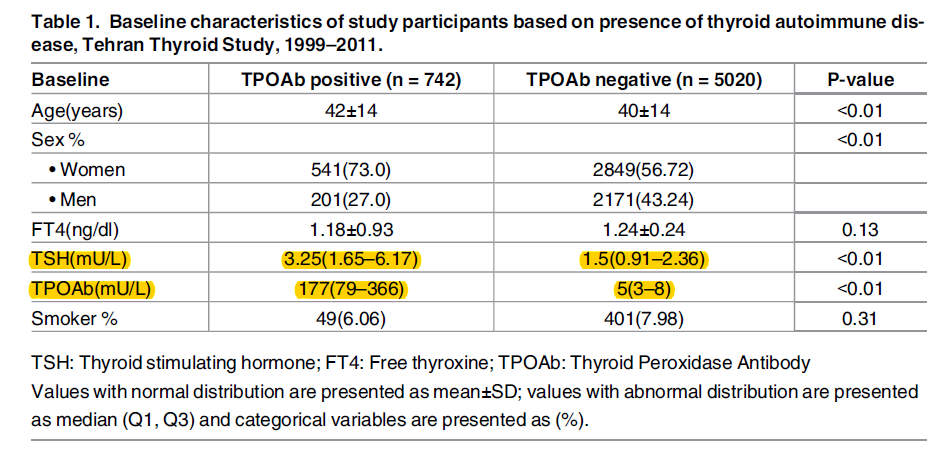
Baseline Observations
- At baseline, those who were TPO positive (TPO=177 in cases vs 5 in controls) had higher TSH (3.25 in cases vs 1.5 in controls)
- At baseline, in the TPOAb positive group:
- 462(62.3%) were euthyroid
- 81(10.9%) overt hypothyroid
- 25(3.4%) hyperthyroid
- Note: What this means is the vast majority who were TPO positive had normal thyroid function. Clinical translation: don’t scare your patients if they have positive TPO antibodies, it suggests a problem may be coming but this is a minimal to moderate suggestion (depending on their levels) and not a guarantee.
Future Risk – when tracking patients over time
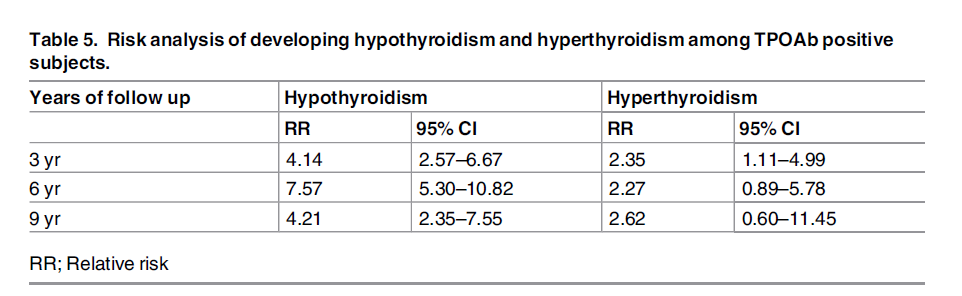
- TPO positive cases were at higher risk for becoming hypothyroid (remember, follow-ups were in 3-year increments)
- We observed that TPOAb positive subjects had a higher risk of developing hypothyroidism in all phases; after 3 years: relative risk [RR] 4.14, after 6 years: relative risk [RR] 7.57, and after 9 years: relative risk [RR] 4.21.
- However, the details here matter. Increased risk could mean a 74% risk or 6% risk, this details matters especially when communicating with patients who tend to revert to a ‘worst case scenario’ thinking.
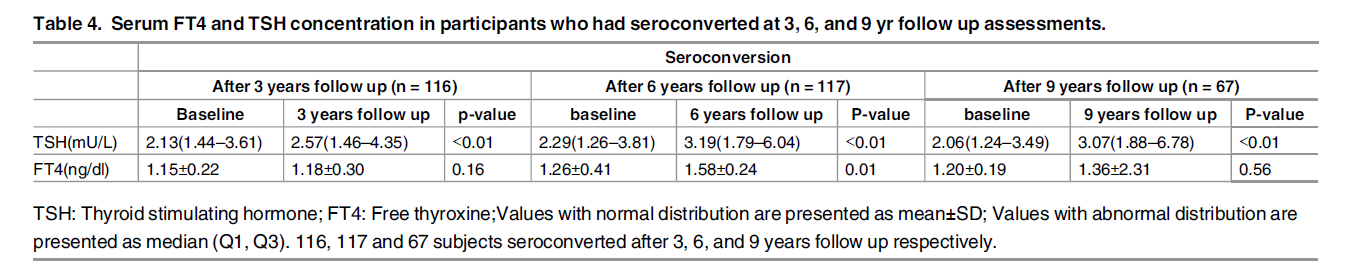
- The table below shows only a small increase in TSH over the 9 year period, wherein the TSH stays well in the normal range. This would suggest a minimal impact on thyroid function.
- Looking at the table below we see increased relative risk (RR) for developing hypothyroidism in those who are TPO positive. But translating relative risk a real world ‘how at risk am I’ is challenging.
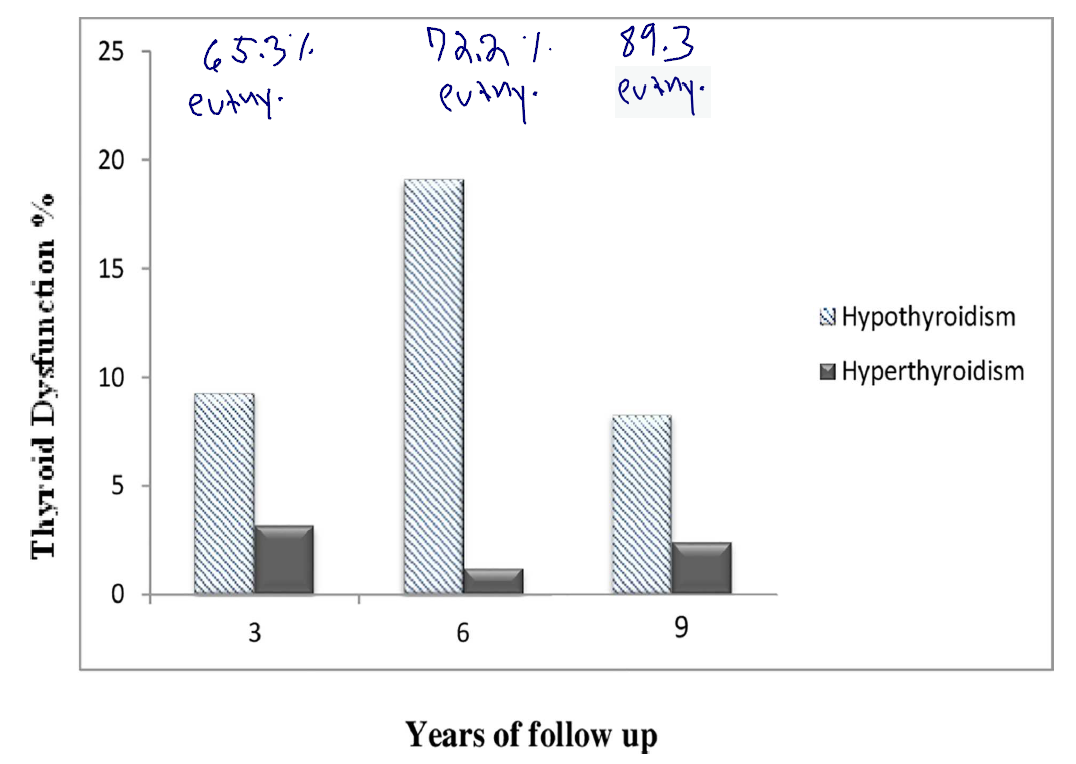
- The graph below helps to answer the question of ‘how at risk am I’ for hypothyroid by tracking the percentage of patients who become hypothyroid over the 9-year follow up period. At 3, 6 and 9 year 65%, 72% and 89% remained euthyroid and only 9%, 19%, and 8% became hypothyroid (normal thyroid= euthyroid, I abbreviated as euthy. below).
- After 3 years follow up, among those who were euthyroid and TPOAb positive (n = 248), 162 subjects (65.3%) remained euthyroid, whereas 23(9.3%) and 8(3.2%) progressed to subclinical and overt hypothyroidism and hyperthyroidism respectively. Likewise, among TPOAb positive euthyroid individuals (n = 162) after 3 years follow up, 117(72.2%) remained euthyroid whereas 31(19.1%) and 2(1.2%) developed subclinical and overt hypothyroidism and hyperthyroidism respectively after 6 years follow up. Finally, of 84 TPOAb positive euthyroid individuals who were euthyroid after 6 years follow up, 75(89.3%) remained euthyroid while 7(8.3%) and 2(2.4%) developed subclinical hypothyroidism and subclinical and overt hyperthyroidism respectively after 9 years follow up.
Other
- The authors suggest that damage may occur in the thyroid gland long before antibodies become positive. Note: not all data support this.
- Also as observed TPOAb positivity was accompanied with increased risk of hypothyroidism, the relationship may be as a result of pathologic changes in the thyroid gland which occurs long before manifestation of TPOAb positivity status.
- Both TSH and TPO predict the development of hypothyroidism
- The Whickham and Busselton cohort study reported that increasing levels of serum TSH, accompanied with TPOAb positivity status, could predict the incidence of overt hypothyroidism.
- The authors list a number of other studies examining the association between TSH, antibodies and predicting hypothyroidism. [12, 28, 8, 4, 30, 9, 10]
Limitations:
- The study population was in Iran and may not fully translate to the US or other countries.
Authors Conclusion:
- “Gender, age and elevated serum TSH were found to be risk factors for developing TPOAb positivity. Furthermore, compared to baseline a significant elevation of TSH levels during seroconversion phase was observed in TPOAb positive individuals.”
- Translation: TSH elevations predicted initiation of thyroid autoimmunity.
Interesting Notes:
- Definitions from this study:
- Euthyroidism was defined as TSH levels with the reference range of 0.32–5.06 mIU/L
- TPOAb levels > 40 IU/mL were regarded as TPOAb-positive
- National Health and Nutrition Examination Survey III (NHANES) reported that over 10% of adults were TPOAb or TgAb positive, with a prevalence of 13% for TPOAb and 11.5% for TgAb.
- In the 20-year Whickham survey, 17% of women and 7% of men developed anti-thyroid antibodies.
Clinical Takeaways:
- There is an association between elevated TSH and TPO antibodies, which appear bi-directional.
- Those with higher TSH are at increased risk of developing thyroid autoimmunity.
- The majority of those with TPO will not have nor develop hypothyroidism, although a clinically relevant % will (perhaps 10-20%). This should be incorporated into the patient education process.
Dr. Ruscio Comments
This study provides some valuable insights regarding thyroid antibodies and the development of hypothyroidism. Yes, there is an association and yes, this is a preventative marker we can look at. However, I fear many practitioners and patients have been led to believe the association between thyroid antibodies and becoming hypothyroid is closer to 100% than the actual 10-20%. We have discussed another recent study reinforcing this concept.
What does this mean? Still act and still educate, but we should be a bit more conservative with how harmful we describe elevations of TPO antibodies to our patients.
A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation.
https://www.ncbi.nlm.nih.gov/pubmed/28315909
Study purpose
- Review the impact of iodine, selenium, vitamin D and gluten on thyroid autoimmunity.
Intervention:
- Review of literature, non-systematic.
Main Results:
Iodine
- “In iodine-replete areas, most persons with thyroid disorders have autoimmune thyroid.”
- “Autoimmune hypothyroidism and thyroid antibodies (TAb) are more common in iodine replete areas than in iodine deficient areas.”
- High iodine intake is also associated with hypothyroidism and other types of thyroid disease
- A number of studies indicated that moderate or mild iodine excess (median urinary iodine >220µg per 24 hours) is associated with a more frequent occurrence of hypothyroidism, especially in elderly subjects.
- Furthermore, a cross-sectional study from south China showed that high iodine intake was likely to lead to the occurrence of thyroid diseases, such as HT, nodular goiter, and hyperthyroidism, through a long-term process.
- Even small increases in iodine intake are associated with an increased prevalence of thyroid autoimmunity [15]. Pedersen et al. [23] found an increased prevalence of TAb, 4-5 years after a cautious salt iodization program…
- The mechanism through which excess iodine causes problem might be due to suppression of autophagy, and through enhance reactive oxygen species production.
- A recent study [24] suggested that the apoptosis of thyroid follicular cells seen in HT development is likely caused by suppression of autophagy activity, which is induced by iodine excess.
- This process is mediated through transforming growth factor-beta1 downregulation, activation of the Akt/mTOR signaling pathway and enhanced reactive oxygen species (ROS) production.
- Iodine Conclusion
- “Considering the above, high iodine supplementation in HT should be discouraged, as not beneficial and possibly harmful. Discouraging iodine over-supplementation must not preclude its appropriate supplementation in pregnancy to a total intake of 250µg/day.”
Selenium
- Selenium essentially functions to reduce inflammation in the thyroid gland
- Selenium supplementation in patients with AITD, including HT, seems to modify the inflammatory and immune responses, probably by enhancing plasma GPX and TR activity and by decreasing toxic concentrations of hydrogen peroxide (H2O2) and lipid hydroperoxides, resulting from thyroid hormone synthesis.
- Selenium aids on conversion of T4 to T3.
- Optimum dietary intake of selenium for its anti-inflammatory effect is 55-75µg/day
- The current recommended dietary intake of Se in adults, in order to achieve the maximal activity of GPX in plasma or in erythrocytes, is between 55 and 75µg per day
- Dietary sources of selenium
- Brazil nuts, oysters, tuna, whole-wheat bread, sunflower seeds, most kinds of meat (pork, beef, lamb, turkey, chicken), mushrooms and rye.
- It does not appear those in the US or Greece are selenium deficient.
- Dr. R’s Note: Most Americans consume adequate amounts of selenium. According to an analysis of data from the 2009–2010 National Health and Nutrition Examination Survey (NHANES), the average daily selenium intake in Americans aged 2 years and older from foods is 108.5 mcg – from the NIH (1)
- In a study conducted in the north-west part of Greece the total daily average intake of Se from the food was 39.3µg per person
- Even though selenium deficiencies are rare, there is clearly benefit from supplementation. 3 meta-analyses have shown the ability of selenium to reduce thyroid antibodies.
- We have previously discussed how selenium appears to have the most benefit between 3-6 months. However, in those on thyroid replacement hormone they may experience benefit from selenium for up to 12 months.
- The recently (2016) published systematic review and meta-analysis of Wichman et al. showed that Se supplementation effectively reduces serum TPO-Ab levels at 3, 6, and 12 months and serum Tg-Ab at 12 months in LT4-treated populations, but not in non-treated ones (5)
- This same study found also that baseline selenium status did not predict effect from supplementation
- However, no significant correlation between the baseline serum Se and the decrease in serum TPO-Ab level was demonstrated in LT4-treated patients.
- And that selenomethionine outperformed sodium selenite. This is one area where the more expensive, functional-medicine-preferred supplement, is more effective
- This meta-analysis also showed a significant decrease in serum TPO-Ab levels in the patients groups receiving 200µg selenomethionine, but not in those receiving 200µg sodium selenite
- The difference might lie on the fact that the absorption of selenite is approximately two-thirds of the absorption of selenomethionine
- The authors also cite another meta-analysis via the Cochrane database concluding insufficient evidence to support selenium use. So, the data here are mixed but overall selenium is supported.
- However, another meta-analysis in the Cochrane library concluded that the evidence to support or refute the efficacy of Se supplementation in patients with HT is insufficient (6)
- Regions with lower selenium intake make be at higher risk for thyroid autoimmunity
- A recent population-based study in China [49] provided us with potent circumstantial evidence that low Se intake is associated with thyroid autoimmunity because showed that the prevalence of thyroid diseases (except hyperthyroidism, Graves’ and nodular disease) was higher in a region of low Se intake (serum Se < 69µg/L) compared to a region of adequate Se intake (serum Se >69µg/L)
- Low or high selenium intake may pose risk for type II diabetes, so be cautious not to over supplement with selenium.
- In a meta-analysis of five studies (13.460 participants) a significantly higher prevalence of T2DM was confirmed in patients with relatively low (<97.5µg/L) or high serum Se levels (<132.50µg/L), revealing a U-shaped non-linear dose-response relationship between serum selenium and T2DM [53].
- How to prevent over supplementing? The authors appear to suggest 200mcg/day as an upper limit. Ironically, some signs of selenium overdose might be the same as those of hypothyroidism; hair loss and depression.
- Chronic ingestion of large quantities of Se may have adverse effects in human health [59-64]. Consumption of approximately 330µg of Se per day could be toxic not only for growth hormones and insulin-like growth factor 1 metabolism but also in the synthesis of thyroid hormones [59, 60].
- Possible major side effects include nail and hair loss, anorexia, diarrhea, depression, hemorrhage, liver and kidney necrosis, blindness, ataxia and respiratory disturbances [60, 61, 62, 63, 64].
- Regarding total selenium intake, 50-400mcg/day is safe while 900mcg/day is toxic.
- A Se intake of 50-400µg/d is considered a safe range for adults, while 850-900µg could be allowed as minimum for Se toxicity [65].
Vitamin D
- We have previously discussed a number of trials finding vitamin D can improve thyroid autoimmunity, this paper makes similar comments.
- The authors recommend screening for vitamin D deficiency in Hashimoto’s patients
- Vitamin D might be best given with vitamin K
- concomitant use of cholecalciferol with vitamin K2 (menaquinones) may be necessary [84]
Gluten
- Those with Hashimoto’s should be screened for celiac. Note: this is nothing new
- According to a most recent meta-analysis, all patients with AITD should be screened for CD, given the increased prevalence of the coexistence of these two disorders.
- A diet low in gluten may help those with Hashimoto’s, even in those without celiac. Dr. R’s note: My feeling is a low/lower gluten diet will suffice for many and a full-blown gluten-free diet will only be needed for a minority, and of course for those with celiac. Part of the reason for not doling out fully gluten-free diets is due to cost and difficulty.
- In summary, whereas it is not yet clear whether a gluten-free diet can prevent autoimmune diseases, it is worth mentioning that HT patients with or without CD benefit from a diet low in gluten as far as the progression and the potential disease complications are concerned [98].
- Still, a lifelong gluten-free diet is not easy to maintain, it could be very costly and the subject’s quality of life may deteriorate [99].
- Also remember in a previous edition of the Review we discussed that only 9% of those with NCGS had thyroid autoimmunity, so the association is valid but it is not near 100%.
Authors Conclusion:
- In conclusion, present evidence indicates the beneficial role of diet in the autoimmune status and the clinical course of HT patients. Serum levels of iodine, Se and vitamin D, in HT patients are necessary, and a careful supplementation in case of deficiency of these agents is recommended. Due to the increasing coexistence of HT with CD and other autoimmune diseases, a low gluten diet is important.
Interesting Notes:
- Those who have one autoimmune condition are at risk for others, some which require conventional medical management, which is why patients should continue to see their conventional doctor also.
- In addition, the coexistence of HT with other organ specific diseases [e.g. pernicious anemia, vitiligo, celiac disease (CD), type 1 diabetes mellitus, autoimmune liver disease, primary biliary cirrhosis, myasthenia gravis, alopecia areata, sclerosis multiplex, Addison’s disease], and non-specific [e.g. rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren syndrome, systemic sclerosis, mixed connective tissue disease], non-endocrine autoimmune diseases should be evaluated.
Clinical Takeaways:
- Iodine, selenium, vitamin D, and gluten all play a role in dictating thyroid health.
- Low, or high, iodine intake can be problematic and can be assessed with a 24hr urine test.
- Selenium is likely best used
- at 200mcg/day,
- as selenomethionine,
- for 3-12 month
- High selenium intake can cause some of the symptoms associated with hypothyroidism; depression and thinning hair.
- Supplementing with vitamin D can improve thyroid autoimmunity.
- A gluten-reduced diet may improve thyroid autoimmunity.
Dr. Ruscio Comments
More data is being published documenting the importance of diet and nutrition in thyroid health. It is important to find a progressive yet non-overbearing balance with these recommendations and to bear in mind recommendations here do not need to be absolutist.
Effect of free triiodothyronine concentration on the quality of life of patients treated with levothyroxine.
https://www.ncbi.nlm.nih.gov/pubmed/27129087
Study Purpose
- Essentially, to assess if TSH alone does not provide adequate monitoring for those on T4 thyroid hormone replacement and if an increase in T4 dose could be helpful.
- In view of recent studies showing that LT4 therapy is associated with lower free triiodothyronine (FT3) concentrations despite lower TSH levels, we may hypothesize that TSH monitoring does not fully reflect restoration of thyroid function in those patients. (9)
- examine whether an increase in the dose of LT4, resulting in higher FT3 levels in patients who initially presented with hypothyroid symptoms despite having normal TSH, helps relieve their symptoms
Intervention:
- Prospective, case-control study, n=33.
- TSH, free thyroxine (FT4), and FT3 levels were measured in every patient before and 4 to 6 months after increasing the dose of LT4.
- Blood samples were drawn in the morning, before the ingestion of the patient’s usual LT4 medication
Main Results:
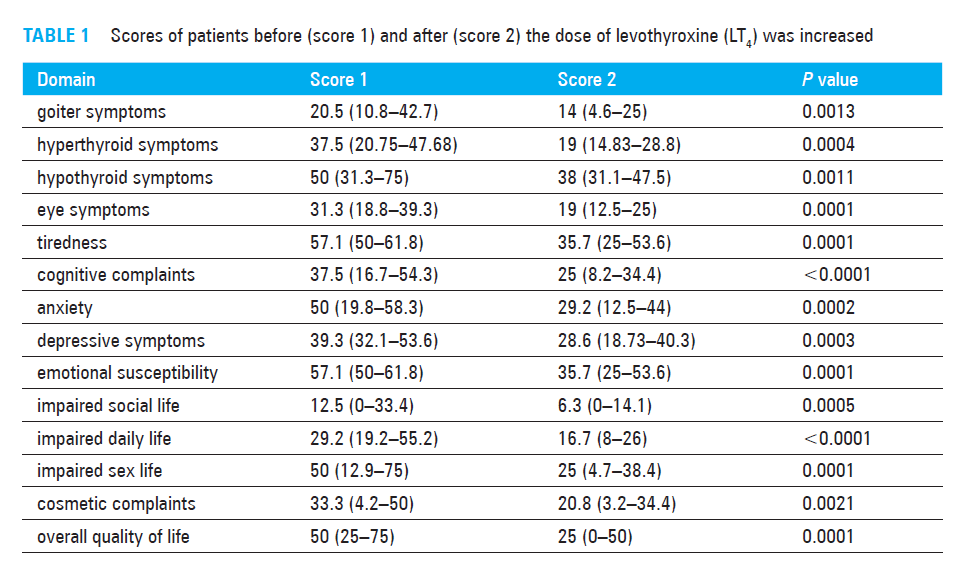
- Here you can see the levels of TSH, fT4, and fT3 at baseline (BL) and after a T4 dose increase (Post) of 79 to 110. As expected TSH decreased while fT4 and fT3 increased. Change in TPO was not recorded.
- The increased dose of T4 correlated with globally improved symptoms.
- Four to six months after the increase in the LT4 dose, scores improved significantly in hypo-thyroid symptoms, anxiety, cognitive complaints, tiredness, depressive symptoms, emotional susceptibility, impaired daily life, impaired social life, impaired sex life, cosmetic complaints, and goiter symptoms
- Four to six months after the increase in the LT4 dose, scores improved significantly in hypo-thyroid symptoms, anxiety, cognitive complaints, tiredness, depressive symptoms, emotional susceptibility, impaired daily life, impaired social life, impaired sex life, cosmetic complaints, and goiter symptoms
- The improved symptoms correlated to either TSH, fT4 or fT3. fT3 appeared to be the most impactful corollary.
- The FT3 concentration before and after the increase of LT4 dose was inversely associated with several QoL outcomes (Spearman’s correlation coefficient test): anxiety (r = –0.3; P = 0.02), depressivity (r = –0.3; P = 0.03), emotional susceptibility (r = –0.5; P = 0.0001), goiter symptoms (r = –0.3; P = 0.01), and tiredness (r = –0.4; P = 0.001). There was also a tendency for the association between the FT3 level and impaired sex life (r = –0.3; P = 0.055). The FT4 concentration was significantly associated with depressivity (r = –0.3; P = 0.02) and cosmetic complaints (r = –0.3; P = 0.006). The TSH level significantly correlated with depressivity (r = 0.25; P = 0.045) and cosmetic complaints (r = 0.3; P = 0.002).
Additional Results:
- The T4 dose increase did not cause adverse events. Dr. R’s note: contrast this with the study we reviewed in June 2018 finding the addition of T3 was prone to causing side effects.
- Interestingly, despite the increase in the dose of LT4, no patients experienced symptoms of overdose
- But don’t some patients do better on T4 plus T3. Yes, however, and as we have discussed prior, this might represent the minority rather than the majority. Today’s paper reinforced that, 1 out of the 33 patients appeared to be a candidate for T4 plus T3.
- In our study, the LT4 dose escalation did not lead to a further increase in FT3 concentrations in 1 patient. Recently, Midgley et al also described this paradoxical effect, explaining that a higher LT4 dose might inhibit the conversion efficiency in some patients. Identifying those patients is important in clinical practice, since they might be candidates for combined T3/T4 therapy.
- Due to potential cardiovascular side effects of increased thyroid hormone dose, the authors do not recommend it in those with cardiovascular disease.
- …dose escalation in patients with cardiovascular diseases is not plausible and reasonable.
Limitations:
- Small sample size
Authors Conclusion:
-
- Essentially the authors feel we should use a tighter TSH reference range for those on T4 and suggest assessing fT3 in patients recalcitrant to T4 therapy.
- Our results might be interpreted in a broader context of the role of TSH measurement in the monitoring of patients on LT4 therapy. Recently, Hoermann et al 5,11 proved that both TSH and FT3 levels are lower in LT4-treated patients, suggesting that the reference range for TSH in this group of patients should be revised. Our findings provide some support for those postulates, and we suggest that the levels of free thyroid hormones should also be measured to assess the adequacy of replacement therapy, particularly in symptomatic patients.
Clinical Takeaways:
- Increasing dose of T4 can improve symptoms in patients recalcitrant to standard T4 dosing
- The improved symptoms correlate with changes in TSH, fT4, and fT3, but most tightly correlate with fT3.
- Increasing dose of T4 might be a better approach, less prone to side effects, than T4+T3 combination therapy.
Dr. Ruscio Comments
This paper findings, when looked at in relation to the previous paper on T4+T3 combination therapy suggest increased T4 dose could be better (less prone to side effects) than T4+T3. While this is not black-white, some inferential evidence suggests that starting with T4 dose increase might be a better strategy. Then, in those who are still symptomatic, consider either testing fT3 or a trail on T4+T3 therapy.
Also, remember there are a few ways to achieve the endpoint of higher serum fT4 and fT3 levels, listed from most to least important.
Increasing fT4 and fT3 levels – treatment hierarchy
- Improve conversion
- Diet, lifestyle and digestive health
- Improve absorption
- Diet, lifestyle and digestive health
- Increase dose of T4
- Potentially assess via more sensitive method, dialysis/LC-MS
- Add T3
- Potentially assess fT3, either via immunoassay or dialysis/LC-MS
As you can see, digestive health is a key factor here. Increased T4 dose and T4+T3 combination therapy also have a time/place, however, they appear lower in the hierarchy than one might think.
Rapid-Fire Research – Ultra-Concise Summaries of Noteworthy Studies
N-acetylcysteine as powerful molecule to destroy bacterial biofilms. A systematic review.
https://www.ncbi.nlm.nih.gov/pubmed/25339490
- NAC has documentation as an anti-biofilm agent.
- “suggested a potential role for NAC as adjuvant molecule in the treatment of bacterial biofilms, with an excellent safety and efficacy profile. NAC, in combination with different antibiotics, significantly promoted their permeability to the deepest layers of the biofilm, overcoming the problem of the resistance to the classic antibacterial therapeutic approach.”
- NAC has been used orally for H. pylori and intramuscular or aerosol for respiratory biofilms.
Female Hormones Influence the Vaginal Microbiota
- “The intravaginal administration of estriol prevents recurrent urinary tract infection in postmenopausal women, probably by modifying the vaginal flora.”
- HRT improves vaginal Lactobacillus colonization, likely secondary to decreased pH.
- Lactobacilli were absent in all vaginal cultures before treatment and reappeared after one month in 22 of 36 estriol-treated women (61 percent) but in none of the 24 placebo recipients (P < 0.001). With estriol the mean vaginal pH declined from 5.5 to 3.8 (P < 0.001), whereas there was no significant change with placebo. (10)
- “The incidence of bacterial vaginosis was significantly lower in the HRT group than in the non-HRT-treated women (5.6% versus 31%)” (11)
Functional 13C-urea and glucose hydrogen/methane breath tests reveal significant association of small intestinal bacterial overgrowth in individuals with active Helicobacter pylori infection.
https://www.ncbi.nlm.nih.gov/pubmed/27586816
- “H. pylori infection was found to be significantly associated with the presence of SIBO as determined by functional breath testing. In addition, SIBO rates appeared to have increased after completed eradication therapies.”
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next practitioner question of the month.

Like what your reading?
Please share this with a colleague and help us improve functional medicine

Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!