Practitioner Research Review – October 2017
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
In Today’s Issue
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however the research studies are a collection of the most clinically meaningful research that has been published recently.
Thyroid Antibodies Interpretation, Selenium Supplementation, and the Gut-Thyroid Connection
Non-published, written by Dr. Ruscio
Dr. Ruscio’s Comments
This is not a published study, but I thought it would be of interest to our Review readers. It is an article I wrote pulling together many important concepts in thyroid care, with supporting references. Enjoy! 🙂
Thyroid Antibodies Interpretation, Selenium Supplementation, and the Gut-Thyroid Connection
Thyroid autoimmunity is the most common autoimmune disorder in westernized countries. A subset of those with thyroid autoimmunity will progress to overt hypothyroidism. Consequently, it is crucial for today’s healthcare provider to be knowledgeable in the area of thyroid so as to successfully manage the conversation around the appropriate care of the thyroid. Perhaps equally as important is appreciating how we strike the right balance of being progressive while also maintaining a degree of reasonability and practicality.
Increased awareness regarding thyroid autoimmunity, thyroid hormone levels, and thyroid hormone metabolism has brought attention to these previously underappreciated areas. However, at the same time, it has created overzealousness that can be detrimental to both patients and healthcare practitioners alike. Let’s discuss important updates in the field of thyroid that can enhance the level of care we provide our patients.
Assessing Thyroid Autoimmunity
Thyroid autoimmunity is the primary driver of hypothyroidism in westernized countries. Thyroid autoimmunity can be assessed via blood testing, most commonly via thyroperoxidase (TPO) and thyroglobulin (TG) antibodies. Elevated levels of these antibodies are often correlated with structural changes in the thyroid gland, which can be detected via ultrasound (hypoechogenicity). If enough damage to the gland has occurred, it can lead to hypothyroidism, defined as high TSH paired with low thyroxine (T4).
TPO antibodies (TPO-Abs) are predictive of subsequent development of hypothyroidism, and the higher the levels, the worse the prognosis. However, the inverse of this observation (i.e., the lower the antibodies, the lower the risk) appears to have eluded many clinicians. Thyroid autoimmunity is not a ‘light switch’ phenomenon; rather, the risk can be stratified according to the degree of elevation of the antibodies. The lower the antibodies, the lower the risk, at least according to the best data available to date.
Interpreting Assays of Thyroid Antibodies
But how do we define a high-risk versus a low-risk level of TPO-Abs? What does the data show? Fear-mongering circles on the internet would have one believe that any elevation is damaging, but this theory is misguided. The most notable study in this regard was published in the journal Hormone and Metabolic Research. [1] Essentially, these researchers found that TPO-Abs below 500 IU/mL posed a minimal risk for being hypothyroid, while TPO-Abs above 500 IU/mL posed a moderate risk of developing hypothyroidism. This suggests that TPO-Abs <500 might be considered a ‘clinical win’ and associated with minimal risk. Translation: otherwise healthy patients with TPO-Abs <500 may not require further treatment to drive down their antibodies.
Some may cite the paper from Brain, Behavior and Immunity in 2012 wherein the authors conclude “TPO-Abs positivity, defined as TPO-Abs >100 IU/mL, significantly predicted poorer psychosocial well-being….” [2] This would suggest that TPO-Abs shouldn’t be >100. But, the devil is in the details. The authors defined a positive TPO-Abs as >100, so they were actually saying those with positive TPO-Abs had poorer psychological well-being. When you look at the data, the average level of TPO-Abs for those who were TPO positive (aka the ‘poorer psychological well-being’ subjects) was 1,122. This further supports that TPO-Abs <500 could be considered a clinical win.
Why all this matters is twofold. One, it will prevent over and unneeded treatment of thyroid autoimmunity. Two, it can prevent fear and stress in the mind of the patient who has already achieved an acceptable level of TPO antibodies.
Selenium Therapy for Hashimoto’s
When discovering they have thyroid autoimmunity, many patients will likely inquire about selenium supplementation as a method for lowering thyroid antibodies. Some studies on selenium supplementation have shown the ability to decrease thyroid autoimmunity, while other studies have not. What do we find when we examine both sides of the data regarding selenium? A 2017 systematic review with meta-analysis [3] found “…evidence to support or refute the efficacy of selenium supplementation in people with Hashimoto’s thyroiditis is incomplete and not reliable to help inform clinical decision-making.”
This is not to say selenium is not therapeutically valuable; it can be. However, upon closer inspection, we see that selenium appears to offer the most benefit when used in the shorter term, from 3 to 6 months. When used beyond that duration, the benefit diminishes below the point of significance according to systematic reviews and meta-analyses. [4] This finding has been reinforced by other systematic reviews with meta-analyses. [4b] What does this tell us? It suggests that selenium has its highest utility when used in the short term, likely for repletion of subtle deficiencies, and does not require long-term dosing. This provides us a practical guideline for selenium prescription, much like the earlier practical approach to addressing thyroid antibodies.
The Gut-Thyroid Connection
While on the topic of selenium, there is one other observation that deserves attention: the relationship between hypothyroidism and small intestinal bacterial overgrowth (SIBO). A recent study that analyzed 1,809 patients found that of all the factors examined (intestinal surgery, acid-suppressing medication use, immunosuppressive drug use…), the two that were most highly associated with SIBO were hypothyroidism and levothyroxine administration. [5] Interestingly, those who were on levothyroxine had a higher risk than those who were hypothyroid. This suggests the association is not simply due to the hypomotility associated with being overt hypothyroid, but perhaps instead has something to do with thyroid disease independent of thyroid hormone levels.
Some evidence has shown that intestinal bacteria utilize selenium and that in deficient states intestinal bacteria may exacerbate selenium deficiency. [6] Speculatively, perhaps those with small intestinal bacterial overgrowth experience a subtle deficiency in their selenium status, which exacerbates a preexisting predilection to thyroid autoimmunity.
This may partly explain why some data has documented improvements in thyroid autoimmunity after administration of antibiotics for H. pylori [7] or Blastocystis hominis [8]. These same antibiotics can treat SIBO, so perhaps the antibiotics are diminishing SIBO, thereby freeing up selenium and thus improving autoimmunity.
In Conclusion
The thyroid is an important area to possess accurate knowledge of because as clinicians we must help our patients filter and contextualize what they read on the internet. It is easy to get swept into an overzealous care model regarding the thyroid. Unfortunately, this does not guarantee better clinical outcomes and oftentimes does the patient a great disservice by subjecting them to unneeded cost, treatment, and worry. By utilizing a progressive yet conservative model for optimizing thyroid function, we can ensure positive patient outcomes with a reasonable degree of time and expense to the patient.
Dr. Michael Ruscio
Folic Acid Therapy Reduces the First Stroke Risk Associated With Hypercholesterolemia Among Hypertensive Patients
https://www.ncbi.nlm.nih.gov/pubmed/27729579
Study Purpose
- Can folic acid reduce stroke risk associated with high cholesterol?
- “We sought to determine whether folic acid supplementation can independently reduce the risk of first stroke associated with elevated total cholesterol levels in a sub-analysis using data from the CSPPT (China Stroke Primary Prevention Trial), a double-blind, randomized controlled trial.”
- NOTE: this study (CSPPT) was in subjects with the MTHFR C677T genotype
Intervention:
- 20,702 hypertensive adults without a history of major cardiovascular disease were randomly assigned to a double-blind daily treatment of
- Enalapril 10-mg and a folic acid 0.8-mg tablet or
- Enalapril 10-mg tablet alone.
- The primary outcome was first stroke.
- Median treatment duration was 4.5 years.
Main Results:
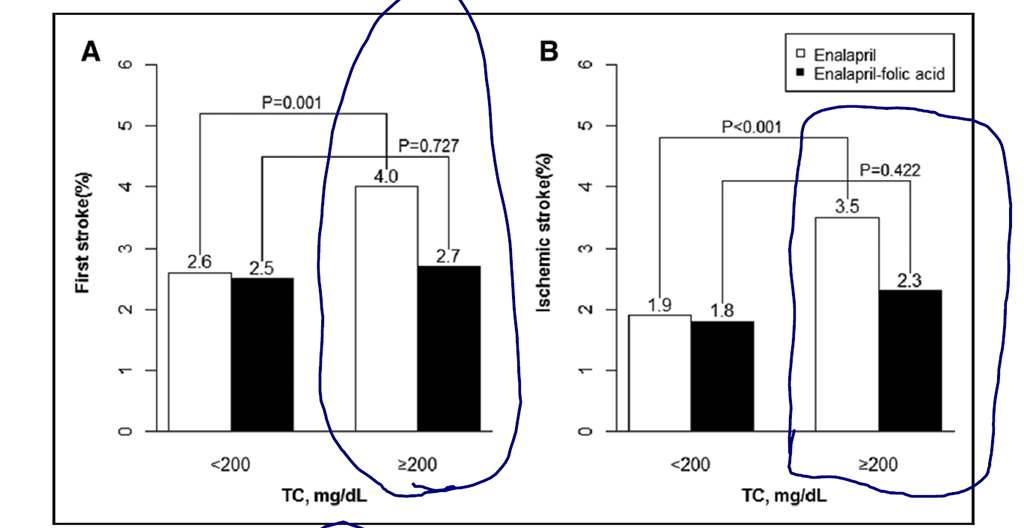
- Folic acid reduced stroke by 31% in those with elevated total cholesterol (>200).
- “This study was the first to demonstrate that elevated TC levels modify the benefits of folic acid supplementation on first stroke. Folic acid therapy significantly reduced the risk of first stroke associated with elevated TC levels by 31%, independent of baseline folate levels and other important covariates.”
- Control (non-folic acid)
- High total cholesterol (≥200 mg/dL) was an independent predictor of first stroke when compared with low total cholesterol (<200 mg/dL).
- Folic acid group
- Folic acid therapy significantly reduced the risk of first stroke among participants with high total cholesterol.
- 4.0% in the enalapril-only group versus 2.7% in the enalapril-folic acid group
- Number needed to treat = 78
- This effect was independent of baseline folate levels and other important covariates.
- Folic acid therapy significantly reduced the risk of first stroke among participants with high total cholesterol.
- Among participants with low total cholesterol, the risk of stroke was 2.6% in the enalapril-only group versus 2.5% in the enalapril-folic acid group. Hence, the effect of folic acid was only noted among participants with elevated total cholesterol.
- The overall results were consistent with ischemic stroke, myocardial infarction, or death from cardiovascular causes.
- Even though folic acid reduced stroke risk, **there was no effect of folic acid on total cholesterol or blood pressure**.
- Similar results were observed between baseline LDL-C levels (≥100 versus <100 mg/dL) and folic acid therapy on incident stroke.
Additional Results:
- NOTE: this study region does not undergo folic acid fortification, so results may not apply to the US and Europe.
- Very few participants were on statins (0.8%), so this study helps to isolate the effects of folic acid.
Authors’ Conclusion:
- “Elevated total cholesterol levels may modify the benefits of folic acid therapy on first stroke. Folic acid supplementation reduced the risk of first stroke associated with elevated total cholesterol by 31% among hypertensive adults without a history of major cardiovascular diseases.”
Interesting Notes:
- Maybe folic acid isn’t so bad… It reduces stroke risk by 8% according to a meta-analysis…
- “A meta-analysis of 15 randomized controlled trials found that folic acid supplementation reduced the risk of stroke by 8% on average, but the effect was greater among those trials with a lower percent use of statins, suggesting that the benefits of folic acid supplementation in the prevention of stroke might be hindered by concomitant use of statins because of the possibility of overlapping biological mechanisms.”
- Statins reduce cardiovascular events, but are not tolerated by 10-15% of patients.
- “In the recently published results of the HOPE-3 study (Heart Outcomes Prevention Evaluation 3), treatment with rosuvastatin (10) resulted in a significantly lower risk of cardiovascular events than placebo in an intermediate risk.”
- “…statin intolerance occurs in 10% to 15% of patients (11).”
- “There are >1 billion hypertensive patients worldwide (16) (≈300 million in China (17)). From a public health perspective, in China alone, a 1.3% decrease in absolute stroke risk (number needed to treat=78) associated with folic acid supplementation in patients with hypercholesterolemia could translate into sparing ≈2 million people from stroke >4.5 years.”
- “Furthermore, the beneficial effects were consistent in participants with relatively lower cardiovascular risk, such as younger patients, women, or those with lower glucose levels or lower blood pressure at both baseline and during the treatment period.”
- US residents may also benefit from folic acid.
- “In the United States, after the introduction of folic acid fortification of food, total folic acid intake was only ≈250 to 400 μg/d in women and 300 to 420 μg/d in men (23). From 2003 to 2004, the median serum folate concentration in the United States was ≈11.9 ng/mL (24) (versus 19.9 ng/mL in the CSPPT after treatment). Therefore, we speculate that even in countries such as the United States and Canada, where folic acid fortification and use of folic acid supplements are widespread, there may still be room to further reduce stroke risk by introducing a target-specific folic acid therapy for those individuals with hypercholesterolemia.”
Clinical Takeaways:
- Folic acid, at 0.8 mg per day, can reduce the risk of stroke associated with high total cholesterol in those with MTHFR who are living in non-fortified areas.
- Folic acid did not reduce cholesterol or blood pressure to produce the protective effect on stroke.
- Speculatively, even in the US (which is currently fortified with folic acid) there may be room for benefit in stroke prevention with folic acid supplementation in those with total cholesterol over 200.
Dr. Ruscio’s Comments
Folic acid may have been given a bad rap by nutritionally dogmatic gurus and/or those who speculate from mechanism rather than look to clinical outcome data. This study showed that folic acid can reduce risk of stroke in those with high cholesterol and independent of the MTHFR C677T genotype. The next study we will review provides a sub-analysis to examine if certain MTHFR types experience more or less benefit from folic acid supplementation. There is still more to learn here, so I will remain open and update my opinion as new data become available, but clearly folic acid has shown benefit in this large and well-performed study.
Homocysteine and Stroke Risk Modifying Effect of Methylenetetrahydrofolate Reductase C677T Polymorphism and Folic Acid Intervention
https://www.ncbi.nlm.nih.gov/pubmed/28360116
Study Purpose
- To test the hypothesis that the association between homocysteine and stroke can be modified by the MTHFR C677T polymorphism and folic acid intervention.
Terminology Refresher
- MTHFR 677CC = a common MTHFR genotype with no risk alleles
- MTHFR 677CT = a heterozygous genotype with one risk allele
- MTHFR 677TT = a homozygous genotype with two risk alleles
Intervention:
- “We analyzed the data of 20,424 hypertensive adults enrolled in the China Stroke Primary Prevention Trial.”
- Participants were
- First divided according to their MTHFR status (CC/CT or TT genotype), then
- Treated with, in double-blinded fashion,
- 10-mg enalapril (hypertensive medication) and 0.8-mg folic acid or
- 10-mg enalapril only.
- **folic acid was used, not folate
- The participants were followed up for a median of 4.5 years.
Main Results:
- Control group (enalapril only)
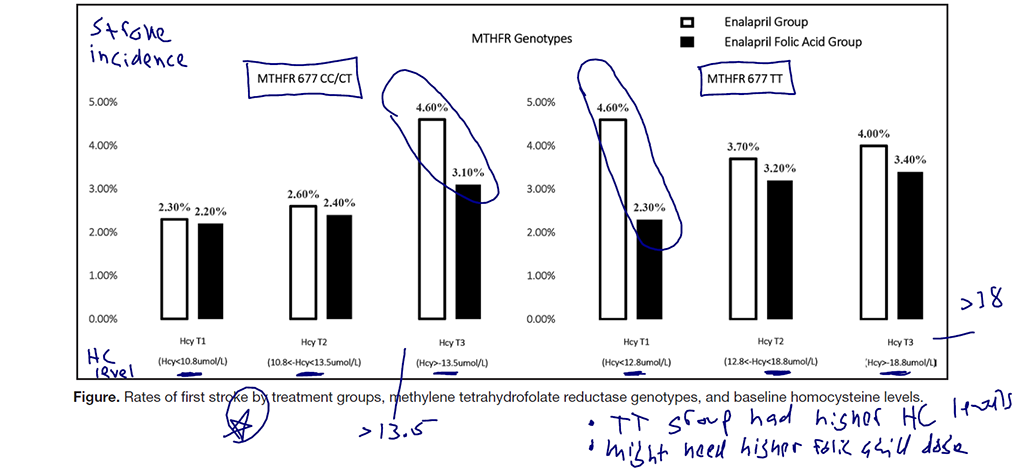
- HomoC levels were associated with stroke in those with the CC/CT genotype, but not the TT genotype.
- Folic acid group
- HomoC showed no significant effect on stroke regardless of genotype.
- Folic acid supplementation significantly reduced stroke risk in participants with CC/CT genotypes and high homocysteine levels.
- Folic acid may not have an effect for those with the TT genotype.
Additional Results:
- Those with TT genotype had higher HomoC and lower folate and B12.
- “Homocysteine levels were higher among those with the TT genotype (≈15 μmol/L) than those with the CC/CT genotype (≈12 μmol/L). Likewise, folate and vitamin B12 levels were lower among those with the TT genotype than those with the CC/CT genotype.”
- Those with TT genotype had a higher relative risk of stroke compared to CC/CT genotypes.
- When comparing the CC/CT to TT groups, an interesting potential trend emerges. The CC/CT groups and TT groups with similar HomoC levels (roughly 13) both improved from folic acid. However, when getting into the higher levels of HomoC that were only seen in the TT group, folic acid no longer had an effect. This may suggest a higher dose would be needed. However, I would expect after 4.5 years, even at a suboptimal dose, an effect would have been realized.
Authors’ Conclusion:
- “In Chinese hypertensive patients, the effect of homocysteine on the first stroke was significantly modified by the methylenetetrahydrofolate reductase C677T genotype and folic acid supplementation.”
Interesting Notes:
- This population does not have fortification of the food supply with folic acid, so this may account for some of the effects.
- “The CSPPT was the first randomized clinical trial to reveal that among hypertensive populations without folic acid fortification, folic acid supplementation can reduce stroke risk by 21%.”
- A meta-analysis has shown that those with MTHFR TT had higher HomoC than those with CC/CT.
- “On average, the MTHFR enzyme loses 70% of its activity in homozygous 677TT.”
- “The characteristics of hypertensive patients in China differ from those of Western populations. More than 50% of hypertensive patients in China have hyperhomocysteinemia, and among these ≈24% carry the MTHFR TT genotype, which is much higher than that of European populations.”
Clinical Takeaways:
- Folic acid supplementation can reduce stroke risk in those living in a non-folic-acid-fortified area.
- Those with the CC/CT MTHFR genotype may have highest benefit.
Dr. Ruscio’s Comments
We have performed an extensive review of the benefit of supplementing with B vitamins to reduce HomoC. We concluded that A) yes, B vitamins do lower HomoC, but B) unfortunately this does not seem to correlate with improved cardiovascular outcomes…. This is likely why this paper has concluded much the same:
“As is well known, folic acid supplementation can lower homocysteine
levels. However, the conclusions of most previous randomized trials have been
negative regarding the impact of folic acid supplementation on stroke risk.”
Alternatively, perhaps the study makes the case that those with the TT genotype and high levels of HomoC (levels above 13 μmol/L) may require methylated folate to realize a HomoC lowering effect. But the effect of this on outcomes has yet to be established, at least to my knowledge. Also, this does not account for why those with a HomoC of 13 μmol/L or below experienced reduced stroke risk from folic acid therapy.
Finally, remember this was done in a Chinese population that does not have folic acid fortification, so this does not mean this would necessarily apply to those in Europe or the US.
This paper was important to highlight that, if for no other reason than to illustrate that for those with various MTHFR mutations (namely CT and TT) wherein folic acid is purported by some gurus to be like poison, folic acid supplementation can actually reduce the outcome of stroke risk. There is clearly more here to learn, so I will remain open and flexible in my opinion as new information emerges.
Mast cells: new therapeutic target in helminth immune modulation.
https://www.ncbi.nlm.nih.gov/pubmed/26577605
Study Purpose
- Review the impact of helminth colonization on mast cells.
Intervention:
- A review of the literature, non-systematic.
Main Results:
- Helminth therapy is being explored as a viable treatment of TH1/TH17-mediated inflammatory disorders, such as multiple sclerosis (10) and inflammatory bowel diseases, including ulcerative colitis (11,12) and Crohn’s disease (13). Human clinical trials to date have demonstrated that this therapy is both safe and effective (10,11,14–16).
- Supporting References
- Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial.
- — 43% improvement rate.
- Effect of hookworm infection on wheat challenge in celiac disease–a randomised double-blinded placebo controlled trial.
- — Did not improve celiac patients’ gluten tolerance.
- Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study.
- — May aid MS.
- Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial.
- Supporting References
- Despite these encouraging findings, a large clinical trial in Crohn’s disease did not show benefit and was stopped.
Additional Results – Encouraging Findings
- Populations with higher colonization rates of helminths show lower levels of inflammatory conditions like IBD.
- Initial clinical reports have shown helminths to be safe.
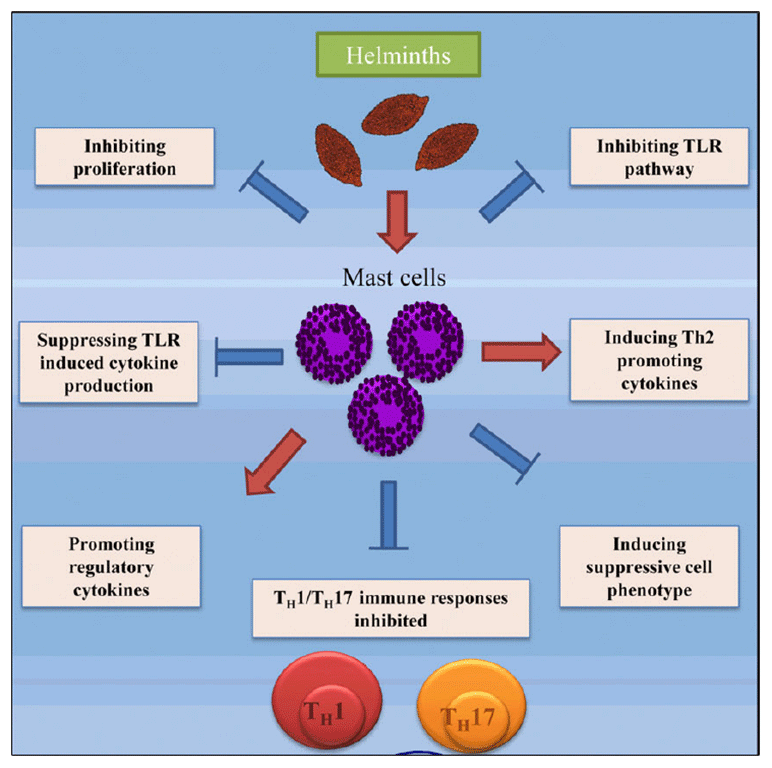
- Some evidence has shown helminths can inhibit mast cell degranulation (note: this could lower histamine).
- Diagram highlighting mechanisms of helminths:
Additional Results – Concerning Findings
- Helminth colonization can increase the number of mast cells at colonization site.
- Helminths may cause GI upset, however this may be short-term.
- Helminth colonization may induce the release of inflammatory mediators like histamine, and may skew immune systems toward Th2.
Authors’ Conclusion:
- “It is therefore possible that the protective effect of helminths against immune disorders may be the result of its molecules directly blocking the release of pro-inflammatory mediators from mast cells. These findings might lead to the development of a therapeutic inhibitors for pathogenic mast cell phenotypes.”
Clinical Takeaways:
- Helminths may aid in certain inflammatory disorders, but we still have much to learn here.
Dr. Ruscio’s Comments
One thing here that struck me is if you look only at mechanism, you can quickly become confused. Some mechanisms suggest helminths should exacerbate inflammation (increased mast cells, increased inflammatory mediator release), while others suggest helminths may decrease inflammation (inhibition of mast cell degranulation)… This is why a clinical argument should not be made from mechanism data, and we should look to clinical trials. If not, we are guessing. Sometimes this is OK and we as clinicians are forced to make the best guess we can. However, I continue to emphasize this point because functional medicine marketers love to use mechanism to spin support for whatever they believe or are selling while overlooking clinical outcome data.
I am curious as to whether helminth therapy can help a subset of patients who appear highly sensitive and reactive and who do not respond fully to other therapies. This group could partially be labeled as highly histamine sensitive or even as MCAD (mast cell activation disorder). In a coming podcast, we will explore this. It is also something I am just starting to experiment with in the clinic.
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next Practitioner Question of the Month.

Like what you’re reading?
Please share this with a colleague and help us improve functional medicine.
Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!