Practitioner Research Review – August 2018
Dr. Michael Ruscio’s Monthly – Future of Functional Medicine Review Clinical Newsletter
Practical Solutions for Practitioners
In Today’s Issue
Research
- A concise, practical guide to diagnostic assessment for mast cell activation disease
- A Paleolithic-type diet results in iodine deficiency: a 2-year randomized trial in postmenopausal obese women
- Exercise Modifies the Gut Microbiota with Positive Health Effects
- Rapid-fire Research – ultra concise summaries of noteworthy studies
- Functional 13C-urea and glucose hydrogen/methane breath tests reveal significant association of small intestinal bacterial overgrowth in individuals with active Helicobacter pylori infection.
- Differences of microbiota in small bowel and faeces between irritable bowel syndrome patients and healthy subjects.
- Unfavorable Course of Subclinical Hypothyroidism in Children with Hashimoto’s Thyroiditis Compared to Those with Isolated Non-Autoimmune Hyperthyrotropinemia.
- Tablet and oral liquid L-thyroxine formulation in the treatment of naïve hypothyroid patients with Helicobacter pylori infection.
- Retrospective Study of Patients Switched from Tablet Formulations to a Gel Cap Formulation of Levothyroxine: Results of the CONTROL Switch Study.
Research
*Please note: the case study and research studies are not meant to be mutually reinforcing. There is often concept overlap, however the research studies are a collection of the most clinically meaningful research that has been published recently.
A concise, practical guide to diagnostic assessment for mast cell activation disease
https://www.wjgnet.com/2218-6204/full/v3/i1/1.htm
Study purpose
- Provide an overview for diagnosing MCAD and MCAS (mast cell activation disease/syndrome)
- Dr. Afrin, a 2 time guest on the show, is one of the co-authors [1, 2]
Intervention
- Expert commentary
Main Results:
- Obtaining definitive, objective confirmation of MCAS is difficult and lack of objective evidence should not preclude empiric treatment (I am inferring slightly).
- Whether assessing for mastocytosis or MCAS, testing is fraught with potential pitfalls which can easily yield false negatives leading to erroneous rejection of diagnostic consideration of MCAD in spite of a clinical history highly consistent with MCAD.
- MCAS falls underneath an umbrella of MCAD. MCA disease is rare while MCA syndrome is more common – iceberg phenomena
- “Of hematopoietic origin, mast cells (MCs) are found in all human tissues, especially at the environmental interfaces and perivascular/perineural sites.”
- MCAS may be caused, in part, by genetic mutations. Some occurring at birth and some accruing over time.
- Although not yet independently confirmed, there soon followed provocative, repeated findings from one group of a very wide array of (presumably mostly constitutively activating) mutations scattered across all domains of KIT in small cohorts of MCAS patients [19,20]. Many of these patients appeared to bear multiple mutations in MC KIT.
- Clinical correlation suggests acquisition of the initial disease-causing mutations occurs relatively early in life, but ad-additional mutations may develop in subclones over time.
- Dr. R’s note: If you experiment with MCAS treatments with your patients and are unable to achieve improvement, I would then refer to an MCAS specialist because a patient may require a highly customized treatment plan (which might be most efficiently constructed with a specialist guidance)
- some MCAS patients benefit little from the first few or several MC-targeting therapies tried, risking premature rejection of the diagnosis.
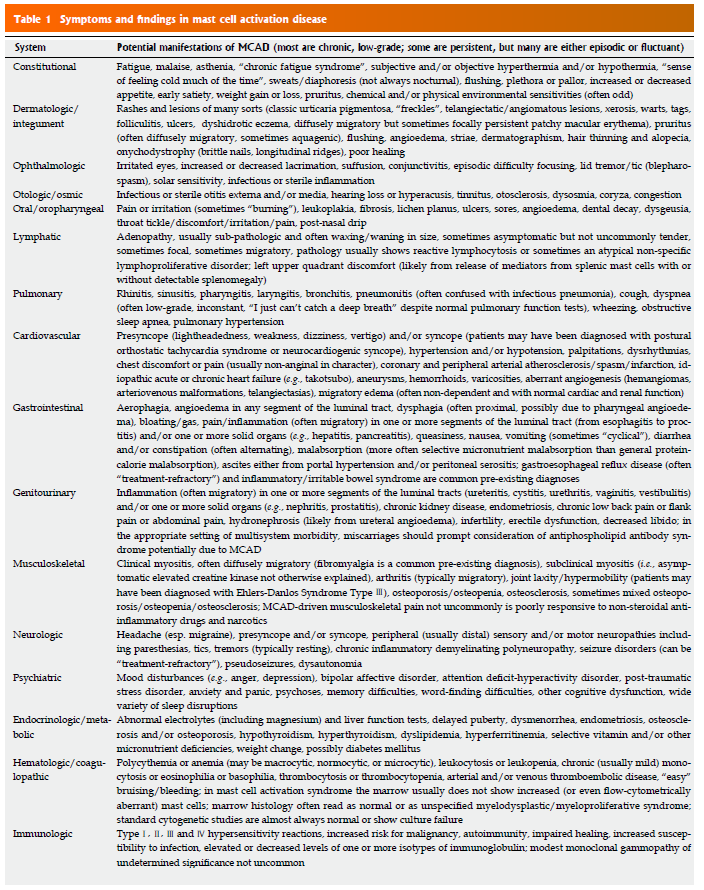
- Since testing can be ambiguous, “It is reasonable to suspect MCAD when at least several symptoms and signs of MC activation are present (Table 1) and no other diagnosis better accounting for the full range of findings is present.”
- A CBC might provide some initial insight, but don’t count on it
- Frequent, though not necessarily constant, relative or absolute monocytosis, eosinophilia, basophilia, and/or reactive lymphocytosis, typically to just modest degrees, can be seen along with similar patterns of abnormality in routine chemistries such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP) (LBA and GJM, unpublished data), hyperbilirubinemia, and diet-independent hypercholesterolemia and hypertriglyceridemia
- it is a confounding point for hematologists that this disease of fundamentally hematologic classification often presents with not a single abnormality in the CBC or differential
- Serum tryptase is not a reliable marker for MCAS
- It is now understood that the serum total tryptase level much more reflects the total body MC load than the total body MC activation state [40-42]. As such, serum tryptase is expected to be elevated in mastocytosis but usually is elevated little to none in MCAS.
- Afrin et. al do offer a diagnostic algorithm, but I find it to be unpractical for those not specializing in MCAS.
- Afrin does provide a questionnaire that I have been finding quite useful in quantifying the patient risk of MCAS. *please see full text for the questionnaire.
- Figure 4 Validated questionnaire to recognize symptoms as part of a mast cell activation disease in a standardized manner (modified from [39,119]). The indicated values for those items acknowledged by or found in the patient are summed. A total score above 8 but less than 14 indicates a pathological activation of mast cells. At a total score of 14 and more, a systemic mast cell mediator release syndrome is clinically verified
- Dr. R’s note: There are nuances regarding testing for MCAS and because of this I would not recommend performing the testing yourself but rather leaving this to an MCAS specialist. This testing could cost a few thousand dollars for the patient, I wouldn’t risk not performing the testing properly.
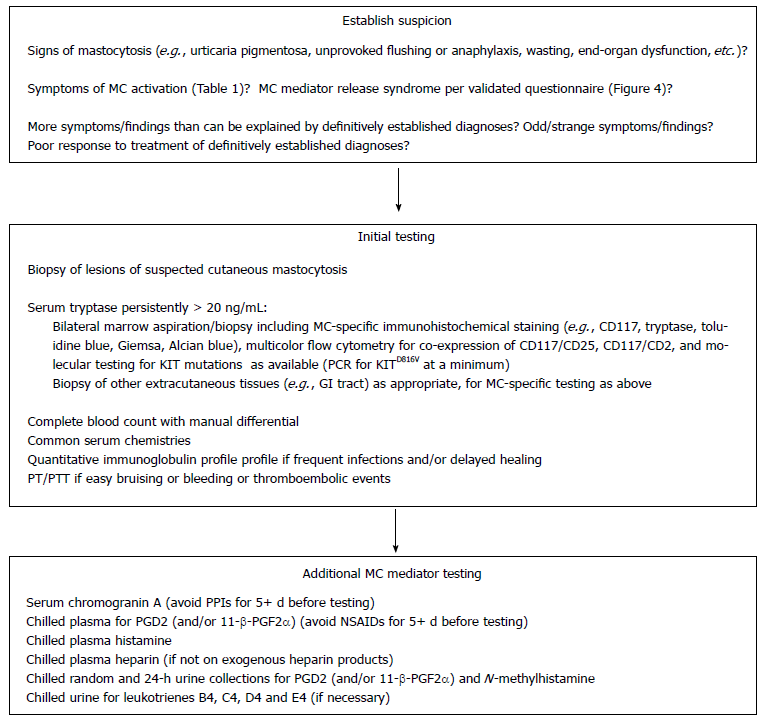
- In the authors’ opinion, such an initial mediator survey for evidence of MCAS should include serum chromogranin A; chilled plasma for prostaglandin D2 (PGD2) (and/or 11-β-PGF2α); chilled plasma histamine; chilled plasma heparin (in patients not on exogenous heparin products); and chilled random and 24-h urine collections for PGD2 (and/or 11-β-PGF2α) and N-methylhistamine [26]. In some situations (see below), urinary levels of leukotrienes B4, C4, D4 and E4 may also be worth pursuing [26,67].
- Although specimen collection as soon as possible following an acute flare of symptoms is ideal, there is no need to wait for such an event when initially assessing the patient with longstanding baseline symptoms consistent with aberrant MC mediator release. However, if the initial laboratory assessment in a patient with a history suspicious for MCAD is negative, repeat testing is usually warranted but preferably should be deferred until the presentation of an acute flare. If possible, hourly determinations of serum tryptase, plasma PGD2 and histamine, and spot urinary PGD2 and N-methylhistamine should be pursued at baseline and over the next 2-3 h as a flare evolves.
- When repeated efforts to identify aberrant MC activation using the above-described screening approach all fail, consideration can be given to screening for aberrant expression of less specific MC mediators such as Factor Ⅷ[64,101], plasma free norepinephrine [102], tumor necrosis factor alpha [103-111] and interleukin-6 [103].
Clinical Takeaways:
- MCAS is more common than MCAD and should be a consideration in otherwise non-responsive patients
- Start with the gut and basic histamine reduction strategies
- If these fail, consider using Afrin’s questionnaire and then referring to an MCAS specialist
Dr. Ruscio Comments
Here is how I would approach the suspicion of MCAS.
1st Do everything you can for gut health and histamine intolerance (diet, fasting, dysbiosis +/- a low histamine diet)
2nd Experiment with direct antihistamine therapies (vitamin C, glutamine, herbal/vitamin anti-histamine blends, OTC H1/2 antagonists)
3rd Have patient fill out MCAS questionnaire
4th If over 8, consider referring to an MCAS specialist, if over 14, strongly consider referring to an MCAS specialist
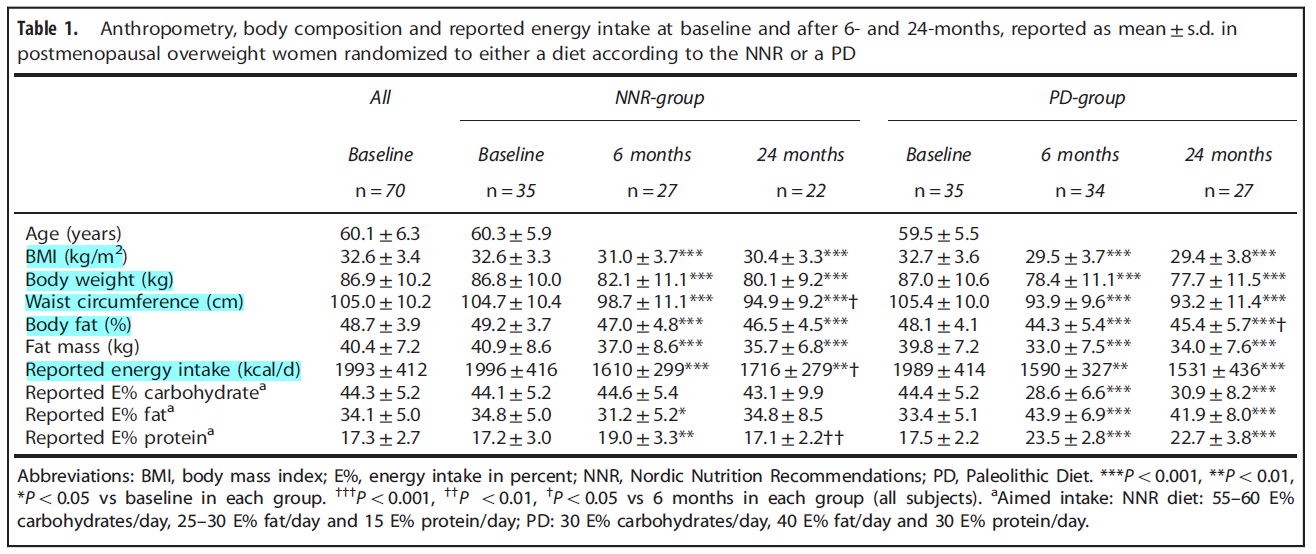
A Paleolithic-type diet results in iodine deficiency: a 2-year randomized trial in postmenopausal obese women
https://www.ncbi.nlm.nih.gov/pubmed/28901333
Study purpose
- To assess the impact of a paleo diet on iodine status. Note: “two of the largest iodine sources, table salt, and dairy products, are excluded.”
Intervention:
- A 2-year prospective randomized trial
- Comparing 24-hour urinary iodine concentration (UIC) in those on a paleo diet with those on the Nordic Nutritional Recommendations (standard dietary advice in Norway).
- Dietary iodine intake, 24-UIC, 24-h urinary iodine excretion (24-UIE), free thyroxine (FT4), free triiodothyronine (FT3) and thyrotropin (TSH) were measured at baseline, 6 and 24 months. At each testing interval, 3 separate collections were performed.
- N=49
Main Results:
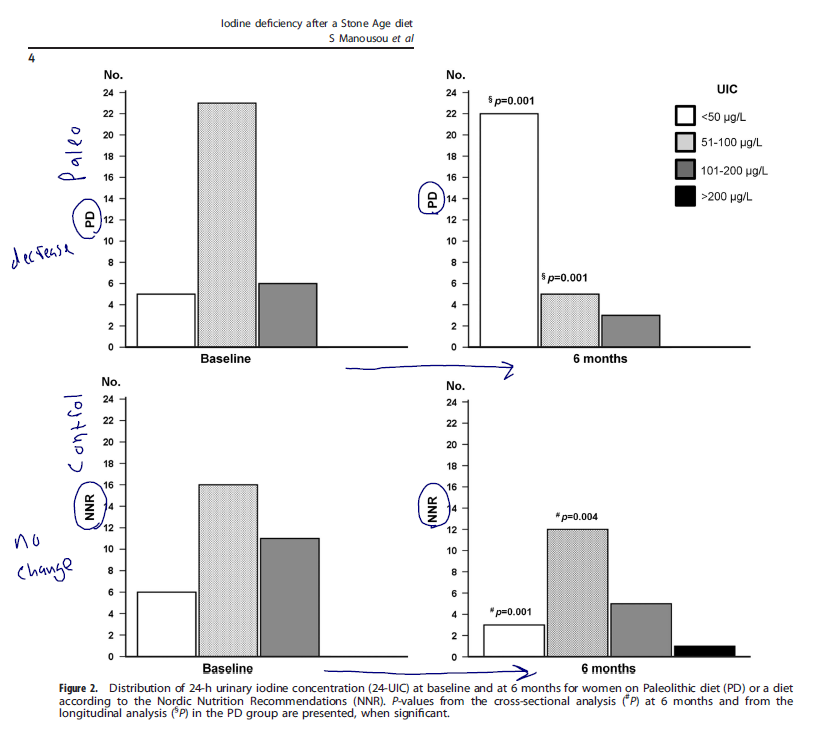
- Summary: the paleo diet lead to a significant reduction in iodine and a transient decrease in free T3 levels (occurring at 6 months then disappearing), while the Nordic diet group did not change.
- At baseline, median 24-UIC (71.0 μg/l) and 24-UIE (134.0 μg/d) were similar in the PD and NNR groups.
- After 6 months, 24-UIC had decreased to 36.0 μg/l (P = 0.001) and 24-UIE to 77.0 μg/d (P = 0.001) in the PD group; in the NNR group, levels were unaltered.
- FT4, TSH and FT3 were similar in both groups, except for FT3 at 6 months being lower in PD than in NNR group.
- The paleo diet led to better results in weight loss. Dr. R’s note: we have discussed previously that one noteworthy study found that subjects on the paleo diet experienced improved mood, energy, and body composition while simultaneously seeing a decrease in fT3 levels – so don’t be fooled into thinking that a reduction within conventionally normal range of fT3 is a bad thing. *I could not locate the reference on this one guys, but it is on our site somewhere*
- The PD-group decreased more in weight (mean 8.6 kg (9.9% from baseline)) during the first 6 months than the NNR-group (4.7 kg (5.4% from baseline; P = 0.001)).
- After 24 months, the total weight loss from baseline was 10.7% in the PD-group and 7.7% in the NNR-group (P = 0.08)
- Clinically, the shift in iodine and free T3 may not matter.
- At 6 months, thyroid function hormones in the PD group were unaltered compared to baseline, although iodine was at its lowest level, which was consistent with mild ID.
- Thyroid function hormones in mild ID are not affected in vegans and vegetarians with a large difference in spot UIC but similar FT4 and TSH [10]
- With one exception, pregnant women or those trying to become pregnant.
- The low iodine content in a PD may have adverse effects, especially if the diet is used by fertile women.
Additional Results:
- Changes in iodine-containing foods. Paleo dieters increased fish consumption while decreasing dairy, bread, cereals. Nordic diet remained constant.
- After 6 months, subjects on PD had doubled reported intake of foods containing fish, seafood, spawn and caviar from 46.1 to 97.1 g/day. Simultaneously, the PD group reduced intake of dairy products from 269 to 7.4 g/day and cheese from 28.9 to 1.6 g/day. Other foods were also reduced in the PD group after 6 months: bread from 99.2 to 5.8 g/day, cereals from 68.8 to 0.64 g/day and ice cream from 12.5 to 0.68 g/day. The changed dietary pattern was sustained at 24 months (data not shown). In the NNR-group, the intake of iodine-containing foods remained constant (data not shown).
- Salt intake remained constant.
- salt intake, estimated by 24U-Na, did not differ between groups
Authors Conclusion:
- “A PD results in a higher risk of developing ID, than a diet according to the NNR. Therefore, we suggest iodine supplementation should be considered when on a PD.”
Interesting Notes:
- What is the best method for assessing iodine status?
- “…the best way of determining iodine status is by calculating the 24-h urinary iodine excretion (24-UIE) [17] over multiple days, as daily iodine intake varies.”
- The paleo diet has been shown effective for weight loss, metabolic balance and triglyceride levels, both in the short and long term.
- In short-term studies [2,3], a Paleolithic-type diet (PD) has been beneficial for weight reduction and metabolic balance. Positive long-standing effects on fat mass, abdominal obesity and triglyceride levels are also reported [4].
- Dietary parameters
- The PD aimed at providing 30 energy percent (E%) protein, 40 E% fat and 30 E% carbohydrates. Lean meat, fish and seafood, fruit, vegetables, root vegetables, eggs, and nuts were included in the PD, whereas, dairy products, cereals, beans, refined fats and sugar, added salt, bakery products and soft drinks were excluded. Processed, canned and preserved food, in addition to manufactured and semi-manufactured goods, were avoided.
- The NNR diet aimed for an intake of 15 E% protein, 25–30 E% fat and 55–60 E% carbohydrates; that is, lower protein and fat content and higher carbohydrate content than in the PD. The diets were consumed ad libitum during the 2-year study.
Clinical Takeaways:
- A paleo diet tends to be more effective for metabolic health than normal reference diets
- A paleo diet may lead to a subclinical deficiency of iodine, which is most relevant for women who are trying to become or are already pregnant
- This change in iodine intake may cause a transient decrease in free T3 which appears clinically insignificant
Dr. Ruscio Comments
I have theorized previously that part of the reason the Paleo diet appears beneficial for those with Hashimoto’s and hypothyroid, at least in part, is the reduction of dietary iodine secondary to reduced consumption of dairy, gluten and iodized table salt (see two episodes here 1 and 2). A handful of studies have essentially found a reduction in iodine intake can benefit thyroid function and autoimmunity. So, the reduced levels of iodine might not be a bad thing. However, it may be prudent to retain a reasonable amount of iodine intake in the diet, perhaps 150-450 micrograms per day.
It is also important to mention that a reduction in free T3 appears to accompany healthy metabolic stress like reduced calories, fasting or as in this case reduction in carb intake. This metabolic stress leads to improved health of the host, seen here as reduced weight. So the reduced fT3 should not be viewed as a bad thing but rather as a normal shift in hormones seen secondary to a healthy metabolic stressor.
A simple strategy to maintain some iodine in your diet could be to cut your sea salt with a sea salt + iodine-rich sea vegetable seasoning salt. I combine 2/3 sea salt + 1/3 sea salt with sea vegetables. Also, remember at first glance the result of this study appear disheartening however upon closer examination the impact appears to be clinically irrelevant, unless pregnant or attempting to become pregnant.
Exercise Modifies the Gut Microbiota with Positive Health Effects
https://www.ncbi.nlm.nih.gov/pubmed/28357027
Study purpose
- To expand upon the positive effects exercise exhorts upon the microbiota
- Recent studies suggest that exercise can enhance the number of beneficial microbial species, enrich the microflora diversity, and improve the development of commensal bacteria
Intervention:
- Non-systematic review.
Main Results:
- “Recent studies suggest that an increase in exercise can enhance the number of beneficial microbial species and that the microbiota is responsive to the homeostatic and physiological variations due to exercise [5, 11].”
- Exercise may improve gut health via speeding gastrointestinal transit time
- Low-intensity exercise can influence the GIT reducing the transient stool time and thus the contact time between the pathogens and the gastrointestinal mucus layer [5].
- This may be part of the reason why exercise is protective against colon cancer, diverticulosis, and IBD.
- As a consequence, it seems that exercise has protective effects, reducing the risk of colon cancer, diverticulosis, and inflammatory bowel disease [47].
- To paraphrase with some of my own interpretation – excessive exercise may have an ill-effect on GI health, in part due to reduced blood flow to the GI and subsequent leaky gut.
- Conversely, it appears that endurance exercise determines a variation in the GIT due to the reduction of the splanchnic blood flow, as much as 80% of basal levels, resulting in toxicity effects [47, 49].
- Prolonged exercise also determines an increase of intestinal permeability, compromising gut-barrier function and resulting in bacterial translocation from the colon [47, 50].
- Early life exercise might have long-term influences on the microbiota and metabolism
- The authors observed that when exercise started in juvenile period it modified various phyla with an increase of Bacteroidetes and a decrease of Firmicutes [11]. Furthermore, juveniles exercise, compared with adult exercise, modified more genera and led to an increase in lean body mass [11].
- These data suggest that early life exercise can influence the gut microbiota composition stimulating the development of bacteria able to determine adaptive changes in host metabolism [11].
- Mouse model data shows exercise is anti-inflammatory for the gut. Dr. R’s note: likely due to a healthy degree of immunosuppression. Also, remember that too much immunosuppression could be another reason why overtraining may impair gut health.
- They [60] stressed that exercise played an anti-inflammatory action in the gut, although in mice
- Irrespective of diet, cardiorespiratory fitness predicted microbial diversity
- The results demonstrated that, regardless of diet, CRF was correlated with increased gut microbial diversity [71].
- Some have even gone as far to suggest exercise could be used to treat dysbiosis
- Estaki et al. [71] proposed that exercise could be used as a therapeutic support in the treatment of dysbiosis-associated diseases.
Authors Conclusion:
- “…exercise can be used as a treatment to maintain the balance of the microflora or to rebalance eventual dysbiosis, thus obtaining an improvement of the health status.”
Clinical Takeaways:
- Exercise, in the appropriate dose, may improve microbiotal diversity, be anti-inflammatory for the gut, protect against GI diseases and functional as a treatment or preventative measure for dysbiosis.
Dr. Ruscio Comments
This likely goes without saying, but if you are trying to optimize gut health and have not addressed exercise, you are overlooking a foundation piece of the gut health equation.
Rapid-Fire Research – Ultra-Concise Summaries of Noteworthy Studies
Functional 13C-urea and glucose hydrogen/methane breath tests reveal significant association of small intestinal bacterial overgrowth in individuals with active Helicobacter pylori infection.
https://www.ncbi.nlm.nih.gov/pubmed/27586816
- SIBO is more common in those with H. pylori, and SIBO rates may increase after H. pylori eradication.
- H. pylori infection was found to be significantly associated with the presence of SIBO as determined by functional breath testing. In addition, SIBO rates appeared to have increased after completed eradication therapies.
- Note: This is why it is so important to have a holistic view when treating GI dysbiosis. For example, only focusing on SIBO might lead to an incomplete response.
Differences of microbiota in small bowel and feces between irritable bowel syndrome patients and healthy subjects.
https://www.ncbi.nlm.nih.gov/pubmed/26595305
- Those with SIBO were found to have elevated levels of colonic and SI bacteria. Oral bacteria levels were not elevated.
- Higher abundance of colonic Veillonellaceae and SI Prevotellaceae, and lower amount of oral cavity normal flora in proximal SI were found in IBS patients. We may manipulate these bacteria in IBS patients in future studies
- Note: Knowing what type of overgrowth has occurred (upper GI or lower GI) may help guide preventative treatments in the future. Perhaps when smart capsules can cheaply/easily perform multiple samples from the SI. Also, to my knowledge the data showing what type of bacteria overgrow in SIBO are inconsistent.
Unfavorable Course of Subclinical Hypothyroidism in Children with Hashimoto’s Thyroiditis Compared to Those with Isolated Non-Autoimmune Hyperthyrotropinemia.
https://www.ncbi.nlm.nih.gov/pubmed/27914141
- We have previously discussed that the level of antibody elevation in those with SCH predicts the likelihood of development of frank hypothyroidism. This study reinforced these findings with TG antibodies.
- High titers of anti-thyroglobulin antibodies (TGAbs) predicted later medication in the HT group (HRs, 28.2 vs. normal TGAbs, P = 0.013).
- high titers (more than 10 times above the upper normal limit) of TGAbs (P = 0.006) were risk factors for later levothyroxine medication
Tablet and oral liquid L-thyroxine formulation in the treatment of naïve hypothyroid patients with Helicobacter pylori infection.
https://www.ncbi.nlm.nih.gov/pubmed/27848196
- Those with previously eradicated H. pylori responded better to liquid T4 than to tablet T4; TSHs were 1.8 and 3.2 respectively after 9 months. No difference was noted between liquid or tablet in healthy controls.
- At 9 months (after 6 months of Helicobacter pylori eradication) mean thyroid-stimulating hormone values were lower in subjects treated with LT4 tablet (TSH(9mo): TAB = 1.8 ± 1.2 mU/L; SOL = 3.2 ± 1.7 mU/L; p = 0.006).
- On group B (controls) no difference were observed, at each time point, in the mean thyroid-stimulating hormone values and thyroid-stimulating hormone variations between two LT4 formulations.
- LT4 liquid formulation may produce a better clinical response compared to the tablet formulation in hypothyroid subjects with Helicobacter pylori infection.
- Note: This has significant clinical implications. It suggests that those with GI infections may require more absorbable, liquid, forms of thyroid hormones.
Retrospective Study of Patients Switched from Tablet Formulations to a Gel Cap Formulation of Levothyroxine: Results of the CONTROL Switch Study.
https://www.ncbi.nlm.nih.gov/pubmed/27943146
- How often do normal hypothyroid patients need or prefer liquid thyroid hormone?
- This study suggests 49% will have better TSH and 62% will have better symptomatic control
- Of the 99 patients studied, the majority (51.5%) experienced no documented change in TSH status after the switch (P < 0.0001). However, there was a decrease in the mean number of dose changes experienced (1.61 ± 0.96 vs. 0.73 ± 0.96; P < 0.0001). Improved hypothyroid symptom control was reported among 61.6% of patients (61 of 99; P < 0.0001).
- Perhaps we could construct the following thyroid hormone hierarchy
- 1st – stand/tablet LT4 trial, with dose adjustments + optimize gut health/general health
- 2nd – considerably higher than normal LT4 dose, perhaps guided by dialysis/LC-MS, targeting TSH in lowest ½ of the acceptable range
- 3rd – depends on history/context
- consider a trial on hypoallergenic tablet LT4 (Nature Thyroid, WP Thyroid) – levels responding but symptoms not, and/or reactions occurring
- or, trial on liquid LT4 – levels not fully responding
- 4th – consider the trial addition of T3
I’d like to hear your thoughts or questions regarding any of the above information. Please leave comments or questions below – it might become our next practitioner question of the month.

Like what your reading?
Please share this with a colleague and help us improve functional medicine
Discussion
I care about answering your questions and sharing my knowledge with you. Leave a comment or connect with me on social media asking any health question you may have and I just might incorporate it into our next listener questions podcast episode just for you!